Water is a universal solvent due to its unique polarity and ability to dissolve more substances than any other liquid, a property vital to life on Earth and numerous chemical processes. At WHY.EDU.VN, we explore why this characteristic is essential, delving into the scientific principles that make water such an effective dissolver and how this impacts everything from the environment to human health. Learn about water’s dissolving capabilities, solvency properties, and polar nature.
1. Understanding Water’s Unique Properties
Water’s reputation as the universal solvent stems from its exceptional ability to dissolve a wide array of substances. This capability is crucial for various natural processes, biological functions, and industrial applications. Water’s unique molecular structure and polarity are the key factors that contribute to its solvent prowess.
1.1. Water’s Chemical Composition
Water, chemically represented as H2O, comprises two hydrogen atoms and one oxygen atom. This simple yet unique composition gives water its distinctive properties. The arrangement of these atoms results in a polar molecule, which is fundamental to its solvent capabilities.
1.2. Polar Nature of Water Molecules
The polar nature of water arises from the unequal sharing of electrons between the oxygen and hydrogen atoms. Oxygen is more electronegative than hydrogen, meaning it attracts electrons more strongly. This creates a partial negative charge (δ-) on the oxygen atom and partial positive charges (δ+) on the hydrogen atoms. As a result, water molecules have a positive and negative end, making them polar.
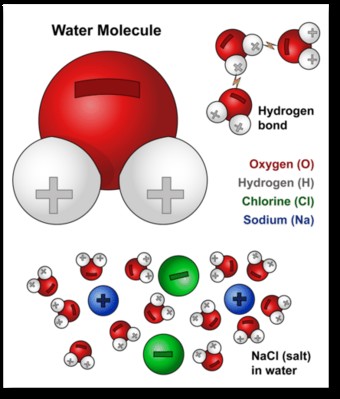 Water molecule with labeled positive and negative charges demonstrating its polar nature
Water molecule with labeled positive and negative charges demonstrating its polar nature
1.3. Hydrogen Bonding
The polarity of water allows it to form hydrogen bonds with other water molecules. The partially positive hydrogen atom of one water molecule is attracted to the partially negative oxygen atom of another. These hydrogen bonds are relatively weak compared to covalent bonds, but they are numerous and collectively strong, giving water many of its unique properties, including its high surface tension and boiling point.
2. The Science Behind Water’s Solvent Ability
Water’s ability to dissolve substances is rooted in its polar nature and its capacity to interact with other polar and ionic compounds. This interaction disrupts the attractive forces holding the solute molecules together, allowing them to disperse evenly throughout the water.
2.1. Dissolving Ionic Compounds
Ionic compounds, such as sodium chloride (NaCl or salt), are held together by strong electrostatic forces between positively charged ions (cations) and negatively charged ions (anions). When salt is added to water, the polar water molecules surround the ions. The oxygen atoms (with their partial negative charge) are attracted to the sodium ions (Na+), while the hydrogen atoms (with their partial positive charge) are attracted to the chloride ions (Cl-).
2.2. Disrupting Ionic Bonds
The attraction between water molecules and the ions of the ionic compound weakens the ionic bonds holding the compound together. This process, known as solvation or hydration, involves water molecules effectively pulling apart the ions and surrounding them, preventing them from reassociating.
2.3. Hydration Shells
Once the ions are separated, they are surrounded by a sphere of water molecules called a hydration shell. This shell isolates the ions and allows them to remain dispersed in the solution. The formation of hydration shells is crucial for the dissolution of ionic compounds in water.
2.4. Dissolving Polar Compounds
Water also dissolves polar compounds, such as ethanol and sugar, through similar interactions. Polar molecules have regions of positive and negative charge, allowing them to form hydrogen bonds with water molecules. This interaction disrupts the intermolecular forces within the solute, leading to its dissolution.
2.5. Solvation of Polar Molecules
The process of solvation for polar molecules involves water molecules surrounding the solute molecules and forming hydrogen bonds with them. This interaction weakens the forces holding the solute molecules together, allowing them to disperse throughout the water.
2.6. Why Water Doesn’t Dissolve Nonpolar Substances
Nonpolar substances, like oils and fats, do not dissolve in water because they lack charged regions that can interact with water molecules. Water molecules are more attracted to each other through hydrogen bonds than to nonpolar molecules. As a result, nonpolar substances tend to aggregate or separate from water, leading to the formation of distinct layers.
3. Significance of Water as a Universal Solvent
Water’s role as a universal solvent has far-reaching implications for various aspects of life and the environment. Its ability to dissolve a wide range of substances is critical for nutrient transport, chemical reactions, waste removal, and numerous industrial processes.
3.1. Biological Importance
In biological systems, water’s solvent properties are essential for transporting nutrients and removing waste products. Blood, which is primarily water, carries oxygen, glucose, and other vital substances to cells throughout the body. It also transports waste products, such as carbon dioxide and urea, to the organs responsible for their elimination.
3.1.1. Nutrient Transport
Water-based solutions are vital for transporting nutrients to cells. Dissolved nutrients, such as vitamins, minerals, and amino acids, can be easily absorbed and utilized by cells for various metabolic processes.
3.1.2. Waste Removal
Water facilitates the removal of waste products from the body. The kidneys filter waste from the blood, and water helps dissolve these substances, allowing them to be excreted in urine.
3.2. Environmental Significance
Water plays a critical role in environmental processes due to its solvent properties. It dissolves minerals from rocks and soil, transporting them to rivers, lakes, and oceans. This process influences the composition of water bodies and affects the availability of nutrients for aquatic organisms.
3.2.1. Weathering and Erosion
Water is a key agent in weathering and erosion. It dissolves minerals in rocks, gradually breaking them down over time. This process releases ions and minerals into the water, which are then transported to other locations.
3.2.2. Nutrient Cycling
Water is involved in nutrient cycling in ecosystems. It transports nutrients from the soil to plants and carries organic matter from decaying organisms back into the environment.
3.3. Industrial Applications
Many industrial processes rely on water’s solvent properties. It is used as a solvent in the production of chemicals, pharmaceuticals, and various other products. Water is also used in cleaning and cooling processes, leveraging its ability to dissolve and carry away impurities and heat.
3.3.1. Chemical Manufacturing
Water is used as a solvent in the synthesis of numerous chemicals. Its ability to dissolve a wide range of substances makes it an ideal medium for chemical reactions.
3.3.2. Pharmaceutical Production
In the pharmaceutical industry, water is used to dissolve and formulate drugs. Its purity and solvent properties are essential for ensuring the efficacy and safety of medications.
4. Factors Affecting Water’s Solvent Ability
Several factors can influence water’s ability to dissolve substances. Temperature, pressure, and the presence of other solutes can all affect the solubility of compounds in water.
4.1. Temperature
Temperature generally increases the solubility of most solid substances in water. As temperature rises, the kinetic energy of water molecules increases, allowing them to more effectively disrupt the attractive forces holding the solute together.
4.2. Pressure
Pressure has a significant effect on the solubility of gases in water. According to Henry’s Law, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. This means that increasing the pressure of a gas will increase its solubility in water.
4.3. Presence of Other Solutes
The presence of other solutes in water can affect its ability to dissolve additional substances. The common ion effect, for example, describes the decrease in solubility of an ionic compound when a soluble salt containing a common ion is added to the solution.
4.4. pH Levels
The pH level of water can influence the solubility of certain substances, particularly those that are acidic or basic. For example, the solubility of calcium carbonate (CaCO3) is affected by pH, with higher acidity increasing its solubility.
5. Water as a Solvent in Human Physiology
Water’s solvent properties are crucial for various physiological processes in the human body. It facilitates nutrient transport, waste removal, and numerous biochemical reactions.
5.1. Digestion and Absorption
Water is essential for digestion and absorption of nutrients. It helps break down food into smaller molecules that can be absorbed by the intestines. Water also transports these nutrients into the bloodstream for distribution throughout the body.
5.2. Kidney Function
The kidneys rely on water’s solvent properties to filter waste products from the blood. Water dissolves these wastes, allowing them to be excreted in urine. Adequate water intake is crucial for maintaining proper kidney function and preventing dehydration.
5.3. Regulation of Body Temperature
Water helps regulate body temperature through sweating. When the body overheats, sweat glands release water onto the skin’s surface. As the water evaporates, it absorbs heat, cooling the body down.
5.4. Cellular Processes
Water is the primary component of cells and is essential for various cellular processes. It acts as a solvent for biochemical reactions, transports nutrients and waste products, and helps maintain cell structure and function.
6. Environmental Impact of Water Pollution
Water’s solvent properties also play a role in the spread of pollutants. Because water can dissolve a wide range of substances, it can easily become contaminated with pollutants from industrial, agricultural, and domestic sources.
6.1. Dissolving Pollutants
Water can dissolve pollutants such as heavy metals, pesticides, and industrial chemicals. These pollutants can then be transported through water bodies, affecting aquatic ecosystems and potentially contaminating drinking water sources.
6.2. Acid Rain
Acid rain, caused by the dissolution of atmospheric pollutants such as sulfur dioxide and nitrogen oxides in rainwater, can have harmful effects on ecosystems. It can acidify lakes and streams, harming aquatic life, and damage forests and soils.
6.3. Eutrophication
Eutrophication, the excessive enrichment of water bodies with nutrients, can lead to algal blooms and oxygen depletion. Water’s solvent properties contribute to eutrophication by transporting nutrients from agricultural runoff and sewage into water bodies.
7. Water Purification Techniques
Given the importance of water quality, various purification techniques are used to remove impurities and pollutants from water. These techniques rely on different principles, including filtration, distillation, and reverse osmosis.
7.1. Filtration
Filtration involves passing water through a filter to remove suspended particles and microorganisms. Different types of filters, such as sand filters and activated carbon filters, are used to remove various types of impurities.
7.2. Distillation
Distillation involves boiling water and then condensing the steam to collect pure water. This process removes dissolved solids, minerals, and microorganisms from the water.
7.3. Reverse Osmosis
Reverse osmosis involves forcing water through a semi-permeable membrane that blocks the passage of dissolved ions and molecules. This technique is effective for removing a wide range of pollutants from water.
7.4. Chemical Treatment
Chemical treatment methods, such as chlorination and ozonation, are used to disinfect water and kill harmful microorganisms. These methods involve adding chemicals to the water to oxidize and destroy pathogens.
8. Case Studies: Water as a Solvent in Real-World Scenarios
Examining real-world scenarios can further illustrate the importance and implications of water’s solvent properties. These case studies highlight the diverse applications and challenges associated with water as a solvent.
8.1. The Flint Water Crisis
The Flint water crisis, which began in 2014, involved the contamination of the city’s drinking water with lead. When the city switched its water source to the Flint River, the water was not properly treated to prevent corrosion of lead pipes. As a result, lead leached into the water supply, causing serious health problems for residents.
8.2. The Deepwater Horizon Oil Spill
The Deepwater Horizon oil spill, which occurred in 2010, released millions of barrels of oil into the Gulf of Mexico. Water’s solvent properties played a role in dispersing the oil, but also in transporting and spreading toxic components of the oil throughout the marine environment.
8.3. Water Treatment in Developing Countries
In many developing countries, access to clean water is limited. Water purification techniques, such as filtration and solar disinfection, are used to treat water and make it safe for drinking. Water’s solvent properties are crucial in these processes, as they affect the efficiency of purification methods.
9. The Future of Water as a Universal Solvent
As the world faces increasing water scarcity and pollution challenges, understanding and managing water’s solvent properties will become even more critical. Innovations in water treatment, conservation, and sustainable water management practices are essential for ensuring the availability of clean water for future generations.
9.1. Innovations in Water Treatment
Ongoing research is focused on developing more efficient and cost-effective water treatment technologies. Nanotechnology, advanced oxidation processes, and membrane technologies hold promise for removing a wider range of pollutants from water.
9.2. Water Conservation Strategies
Water conservation strategies, such as reducing water consumption in agriculture, industry, and households, are essential for preserving water resources. Implementing efficient irrigation techniques, promoting water-saving appliances, and raising awareness about water conservation can help reduce demand and protect water supplies.
9.3. Sustainable Water Management
Sustainable water management practices, such as integrated water resources management (IWRM), aim to balance the needs of different water users while protecting the environment. These practices involve managing water resources in a holistic and participatory manner, taking into account the social, economic, and ecological aspects of water use.
10. Why Choose WHY.EDU.VN for Your Questions?
At WHY.EDU.VN, we understand the importance of having access to reliable and accurate information. Our platform is designed to provide you with comprehensive answers to all your questions, backed by expertise and thorough research. Whether you’re curious about science, history, technology, or any other topic, WHY.EDU.VN is your go-to resource for knowledge.
10.1. Expert-Backed Answers
Our team of experts is dedicated to providing you with well-researched and authoritative answers. We carefully vet all information to ensure its accuracy and relevance, so you can trust the answers you find on WHY.EDU.VN.
10.2. Comprehensive Coverage
We cover a wide range of topics, from scientific principles to historical events. No matter what you’re curious about, you’re likely to find the answer on WHY.EDU.VN. Our content is designed to be informative and accessible, so you can easily understand even complex subjects.
10.3. Easy-to-Use Platform
Our platform is designed to be user-friendly, making it easy to find the information you need. You can search for questions, browse topics, and explore related content to expand your knowledge.
10.4. Community Engagement
Join our community of learners and share your questions and insights. WHY.EDU.VN is a place where you can connect with others who are curious and eager to learn.
11. FAQs About Water as a Universal Solvent
1. What makes water a universal solvent?
Water’s polarity, arising from its molecular structure, allows it to dissolve more substances than other liquids.
2. Why can water dissolve ionic compounds?
Water’s polar molecules attract and disrupt the ionic bonds in compounds like salt, leading to dissolution.
3. How does temperature affect water’s solvent ability?
Generally, higher temperatures increase the solubility of solids in water by increasing the kinetic energy of water molecules.
4. Does pressure impact water’s ability to dissolve gases?
Yes, increased pressure enhances the solubility of gases in water, as described by Henry’s Law.
5. Why can’t water dissolve nonpolar substances like oil?
Nonpolar substances lack charged regions to interact with water, causing them to separate.
6. How does water function as a solvent in the human body?
Water transports nutrients, removes waste, and facilitates numerous biochemical reactions in the body.
7. What role does water play in environmental processes?
Water is essential for weathering, erosion, and nutrient cycling in ecosystems.
8. How do pollutants affect water’s solvent properties?
Pollutants can dissolve in water, leading to contamination and harmful environmental effects.
9. What purification techniques are used to treat water?
Filtration, distillation, reverse osmosis, and chemical treatment are common methods.
10. How can sustainable water management protect water resources?
Integrated water resources management and conservation strategies help balance water needs and protect the environment.
12. Conclusion: Embracing Water’s Solvent Power
Water’s universal solvent properties are fundamental to life and various industrial and environmental processes. Understanding these properties and managing water resources sustainably is crucial for addressing global challenges related to water scarcity and pollution.
12.1. Call to Action
Do you have more questions about water or any other topic? Visit WHY.EDU.VN today to explore our vast collection of expert-backed answers. Our team is ready to provide you with the information you need, whether you’re a student, a professional, or simply curious about the world around you. Join our community and start your journey of discovery with WHY.EDU.VN. Contact us at 101 Curiosity Lane, Answer Town, CA 90210, United States. Whatsapp: +1 (213) 555-0101. Website: WHY.EDU.VN.
Let why.edu.vn be your trusted source for reliable and comprehensive answers.