Why Can Ice Float On Water, a question sparking curiosity across generations? At WHY.EDU.VN, we unravel this fascinating phenomenon, exploring the science behind ice’s unique behavior. Discover why ice, unlike most solids, defies the norm and what makes water so special, examining water density and buoyancy principles.
1. Understanding Buoyancy: The Science of Floating
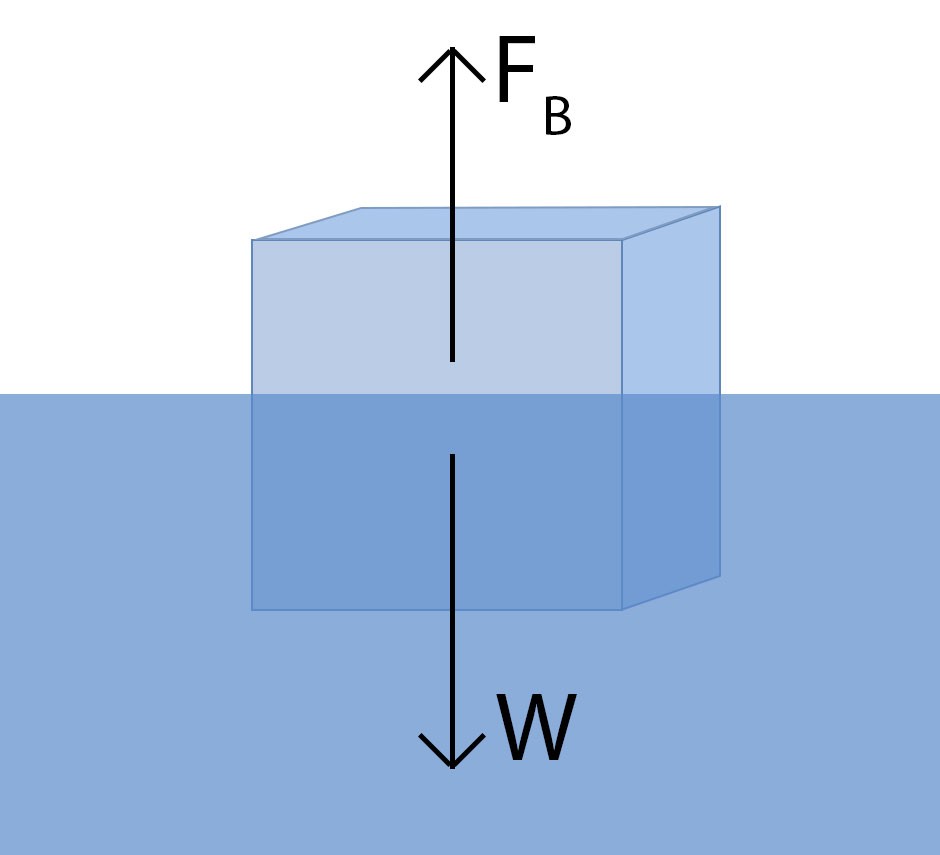
Buoyancy is the force that allows objects to float. Archimedes’ principle is key to understanding this phenomenon. This principle dictates that an object immersed in a fluid experiences an upward buoyant force equal to the weight of the fluid displaced by the object.
Before diving deeper, it’s worth noting that the following explanation involves mathematical concepts. When an object is placed in water, it encounters an upward force, known as buoyant force, opposing gravity. For an object to float either partially or completely submerged, it needs to displace some water, causing the water level to rise.
Archimedes’ principle explains that the upward buoyant force acting on a submerged object is equivalent to the weight of the water displaced by the object. An object’s weight is calculated by multiplying its mass by ‘g,’ representing the acceleration due to gravity. Thus, the upward buoyant force, denoted as FB, is equal to the mass of water multiplied by ‘g.’
Since density is mass divided by volume, we can rearrange this to state that mass equals density multiplied by volume. Therefore, we can express the buoyant force, FB, as the density of water multiplied by the volume of water multiplied by ‘g.’
For an object to float, the buoyant force needs to be at least as strong as the force of gravity. Archimedes’ realization of this principle led to his famous ‘Eureka!’ moment, where he understood that the volume of displaced water is equal to the volume of the submerged portion of the object.
If the volume of water equals the volume of the submerged object, then the buoyant force, FB, is equal to the density of water multiplied by the volume of the submerged object multiplied by ‘g.’
This buoyant force must be at least as strong as the force of gravity acting on the object. The force of gravity is the object’s weight, which is the mass of the object multiplied by ‘g.’ Using the same manipulation, the object’s weight, W, equals the density of the object multiplied by the volume of the submerged object multiplied by ‘g.’
The expressions for FB and W are nearly identical, differing only in the density component. The buoyant force will counteract the force of gravity if the density of the object is less than that of water. This leads to the rule: an object floats on water if it is less dense.
2. Density Differences: Solids vs. Liquids
Usually, solids are denser than their liquid counterparts. A solid object will float on a liquid only if the solid is less dense than the liquid. However, it’s uncommon for a material’s solid form to be less dense than its liquid form.
Materials can exist in different states—solid, liquid, gas—depending on how their particles are arranged. In a solid, molecules are tightly packed in an orderly, repeating pattern known as a crystal lattice.
When a solid is heated, its molecules gain energy and vibrate more vigorously. Eventually, they gain enough energy to break free from their fixed positions in the lattice, transitioning to a liquid state. In this state, molecules move freely but remain close together.
Continued heating allows molecules to break free entirely, forming a gas. Generally, as a material transitions through these phases, its density decreases.
3. The Anomaly of Water: Why Ice is Less Dense
Water is an exception to the rule that solids are denser than liquids. This unique property arises from hydrogen bonding. A water molecule has a V-shape, consisting of an oxygen atom bonded to two hydrogen atoms. These atoms are held together by covalent bonds, where atoms share electrons.
However, the oxygen atom attracts electrons more strongly than the hydrogen atoms, leading electrons to spend more time closer to the oxygen atom. This results in the oxygen end having a slight negative charge and the hydrogen ends having slight positive charges. Opposite charges attract, leading to interactions between water molecules known as hydrogen bonds.
In liquid water, these hydrogen bonds continuously form and break as molecules move. However, as water cools and begins to freeze, it forms a crystal lattice structure. As the molecules attempt to form hydrogen bonds, like charges repel each other, preventing molecules from getting too close. This results in a structure that is less dense than liquid water.
The lower density of ice is due to its molecular arrangement; hydrogen bonds force the molecules into a crystal structure that is more open than the arrangement in liquid water. This explains why ice floats, a phenomenon crucial for aquatic life and climate regulation.
3.1. Hydrogen Bonding Explained
Hydrogen bonding is a type of intermolecular force where a hydrogen atom covalently bonded to a highly electronegative atom such as nitrogen (N), oxygen (O), or fluorine (F) experiences attraction to another electronegative atom in a different molecule or a different part of the same molecule.
3.1.1. The Role of Electronegativity
Electronegativity is a measure of an atom’s ability to attract shared electrons in a chemical bond. Atoms like oxygen are highly electronegative, which means they pull electrons more strongly towards themselves when bonded to hydrogen. This unequal sharing of electrons creates a dipole moment in the molecule, with the electronegative atom carrying a partial negative charge (δ-) and the hydrogen atom carrying a partial positive charge (δ+).
3.1.2. How Hydrogen Bonds Form
In water molecules, the oxygen atom’s high electronegativity results in it having a partial negative charge, while the hydrogen atoms have partial positive charges. When one water molecule comes close to another, the partially positive hydrogen atom of one molecule is attracted to the partially negative oxygen atom of the other. This attraction forms the hydrogen bond.
3.1.3. Strength and Nature of Hydrogen Bonds
Hydrogen bonds are weaker than covalent or ionic bonds but are still significant because of their collective effect in large numbers. They are primarily electrostatic in nature, arising from the attraction between opposite partial charges. The energy of a typical hydrogen bond ranges from 5 to 30 kJ/mol, compared to covalent bonds which are typically 400 kJ/mol.
3.1.4. Impact on Water’s Properties
- High Boiling Point: Water has a relatively high boiling point compared to other similar-sized molecules. This is because extra energy is required to break the numerous hydrogen bonds between water molecules before they can escape into the gas phase.
- High Surface Tension: Hydrogen bonds create a cohesive force that holds water molecules together, resulting in high surface tension. This allows small insects to walk on water.
- Density Anomaly: As water cools, the hydrogen bonds cause the molecules to move further apart, leading to an expansion in volume. This is why ice is less dense than liquid water.
3.2. Crystalline Structure of Ice
The crystalline structure of ice is a critical factor in understanding why ice is less dense than liquid water.
3.2.1. Formation of the Crystal Lattice
When water freezes, the hydrogen bonds between water molecules become more stable and organized. This leads to the formation of a crystal lattice, where each water molecule is hydrogen-bonded to four other water molecules in a tetrahedral arrangement. This arrangement creates a spacious, open structure.
3.2.2. Tetrahedral Arrangement
In the tetrahedral arrangement, each oxygen atom is at the center of a tetrahedron, with hydrogen atoms at two of the vertices and lone pairs of electrons at the other two. This structure maximizes the distance between molecules due to the repulsion between electron pairs and the specific angles of hydrogen bonds.
3.2.3. Open Structure and Increased Volume
The tetrahedral arrangement results in a crystal structure that is more open and less dense than liquid water. In liquid water, the molecules are more closely packed and can move more freely, allowing them to be closer together. When water freezes, the crystal lattice forces the molecules to spread out, increasing the volume by about 9%.
3.2.4. Impact of Impurities
The presence of impurities can disrupt the formation of the perfect crystal lattice, which can affect the density of ice. For example, saltwater ice is denser than freshwater ice because the salt ions interfere with the hydrogen bonding network.
3.3. Density Comparison: Ice vs. Liquid Water
The density difference between ice and liquid water is crucial for understanding the behavior of ice in various environments.
3.3.1. Density of Liquid Water
Liquid water reaches its maximum density at approximately 4°C (39°F). At this temperature, the density of water is about 999.97 kg/m³. Above and below this temperature, the density decreases slightly. The density decreases as the water is heated because the molecules move faster and spread out more, increasing the volume.
3.3.2. Density of Ice
The density of ice at 0°C (32°F) is about 917 kg/m³. This is approximately 9% less than the density of liquid water at 4°C. This difference in density is what allows ice to float on water.
3.3.3. Reasons for Density Difference
- Hydrogen Bonding: As discussed, hydrogen bonding creates an open, spacious structure in ice that increases its volume.
- Molecular Arrangement: The tetrahedral arrangement of water molecules in the ice crystal lattice results in more space between molecules compared to the liquid state.
3.3.4. Environmental Impacts
- Aquatic Life: The fact that ice floats is crucial for aquatic life. When bodies of water freeze, the ice layer forms at the surface, insulating the water below and preventing it from freezing solid. This allows fish and other aquatic organisms to survive the winter.
- Climate Regulation: Ice and snow have high albedo, meaning they reflect a large portion of the sunlight that hits them. This helps to regulate the Earth’s temperature by reflecting solar radiation back into space.
- Geological Processes: The expansion of water upon freezing can cause significant geological changes, such as the weathering of rocks and the formation of ice wedges in permafrost regions.
| Feature | Liquid Water (4°C) | Ice (0°C) |
|---|---|---|
| Density (kg/m³) | 999.97 | 917 |
| Molecular Arrangement | Random, Closely Packed | Tetrahedral, Open Structure |
| Hydrogen Bonding | Dynamic, Fluctuating | Stable, Organized |
| Volume | Lower | Higher |

4. The Limits of Expansion: Can Ice Expansion Be Stopped?
Water is at its densest at approximately 4°C. Cooling it further causes it to expand, and once completely frozen, its volume increases by about 9%. This expansion exerts immense pressure.
The bulk modulus of ice is about 8.8 x 109 pascals. If a full container of water is sealed and frozen, the pressure on the container’s sides reaches approximately 790 megapascals or 114,000 pounds per square inch, equivalent to 7,800 atmospheres. According to Professor Martin Chaplin, a leading expert on water’s properties, no material can withstand such pressures.
4.1. Bulk Modulus of Ice
The bulk modulus of a substance is a measure of how resistant to compression that substance is. It is defined as the ratio of the infinitesimal pressure increase to the resulting relative decrease in volume.
Mathematically, the bulk modulus (K) is expressed as:
K = -V (dP/dV)
Where:
- K is the bulk modulus,
- V is the original volume,
- dP is the change in pressure,
- dV is the change in volume.
The negative sign indicates that an increase in pressure results in a decrease in volume.
The bulk modulus is typically measured in pascals (Pa) or pounds per square inch (psi). A high bulk modulus indicates that the substance is very difficult to compress, while a low bulk modulus indicates that the substance is relatively easy to compress.
4.2. Experimental Evidence and Expert Opinions
Professor Martin Chaplin’s assertion that no material on Earth can withstand the pressures generated by freezing water is based on both theoretical calculations and experimental observations. He and other researchers have conducted numerous experiments to study the behavior of water under extreme conditions.
4.2.1. Compression Experiments
In compression experiments, water samples are subjected to increasing pressure while being cooled. The pressure is typically applied using a hydraulic press or other high-pressure apparatus. The volume and temperature of the water are carefully monitored throughout the experiment.
4.2.2. Observation of Container Failure
One common observation in these experiments is the failure of the containers used to hold the water. Despite using high-strength materials such as steel or reinforced composites, the containers often crack or rupture when the water freezes. This demonstrates the immense pressure exerted by the expanding ice.
4.2.3. Alternative Ice Forms
The idea that water, upon freezing under sufficient pressure, will transform into denser ice forms rather than simply shattering any container is consistent with existing phase diagrams of water and the known behavior of materials under extreme conditions.
5. Freezing Under Pressure: The Formation of Different Ice Types
If water is placed in a strong, rigid container and cooled, pressure increases as more molecules form the lattice structure and press against the remaining liquid molecules. If the container holds, pressure rises rapidly until, at about 200 megapascals (2000 atmospheres), the atoms rearrange into a more compact configuration.
There are 13 known ice forms that are stable at different temperatures and pressures. Ordinary ice is called ice Ih, while the densest high-pressure variety is ice III. In an enclosed container, expansion pressure reaches equilibrium, and the water freezes as a mixture of ice Ih and ice III.
5.1. The Fourteen Known Forms of Ice
The phase diagram of water is complex, with at least 19 known crystalline forms of ice, each stable under different conditions of temperature and pressure. These different forms of ice are typically denoted with Roman numerals.
| Ice Form | Density (kg/m³) | Temperature (°C) | Pressure (MPa) | Key Characteristics |
|---|---|---|---|---|
| Ice Ih | 917 | < 0 | 0.1 | Ordinary ice, hexagonal crystal structure |
| Ice Ic | 922 | < -130 | 0.1 | Cubic crystal structure, metastable at low temperatures |
| Ice II | 1160 | -35 | 200 | Rhombohedral crystal structure, denser than Ice Ih |
| Ice III | 1150 | -25 | 300 | Tetragonal crystal structure, formed under moderate pressure |
| Ice IV | 1170 | -20 | 400 | Metastable, orthorhombic crystal structure |
| Ice V | 1230 | -20 | 500 | Monoclinic crystal structure, stable at high pressures |
| Ice VI | 1310 | 0 | 1100 | Tetragonal crystal structure, denser than Ice V |
| Ice VII | 1650 | 25 | 2500 | Cubic crystal structure, very dense, stable at extremely high pressures |
| Ice VIII | 1660 | -25 | 2000 | Similar to Ice VII but with ordered proton arrangement |
| Ice IX | 1160 | -100 | 200 | Stable at low temperatures, antiferroelectric |
| Ice X | 2500 | High | Very High | Predicted phase, ionic crystal structure |
| Ice XI | 920 | -200 | 0.1 | Ferroelectric, proton-ordered form of Ice Ih |
| Ice XII | 1300 | -50 | 700 | Tetragonal structure |
| Ice XIII | 1240 | -120 | 500 | Orthorhombic structure |
| Ice XIV | 1290 | -150 | 1200 | Orthorhombic structure |
| Ice XV | 1280 | -140 | 800 | Proton-ordered form of Ice VI |
| Ice XVI | 810 | -100 | 0.1 | Zeolite-like structure |
| Ice XVII | Unknown | Unknown | Unknown | Clathrate hydrate filled with hydrogen molecules |
| Ice XVIII | Unknown | Unknown | Unknown | Superionic phase of ice |
| Ice XIX | 1100 | -40 | 350 | Discovered in 2024 |
This table includes key forms of ice and their characteristics, updated with the latest discoveries, such as Ice XIX which was discovered in 2024.
5.2. Formation of Ice III
When water is subjected to high pressure, such as in the depths of the ocean or in laboratory experiments, it can transform into different crystalline forms of ice that are denser than ordinary ice (Ice Ih). One such form is Ice III.
5.2.1. Conditions for Formation
Ice III forms under moderate pressures, typically ranging from 200 to 300 MPa, and at temperatures below the freezing point of ordinary ice. The specific temperature range for Ice III formation depends on the pressure, but it is generally around -25°C at 300 MPa.
5.2.2. Crystal Structure
Ice III has a tetragonal crystal structure, which is different from the hexagonal structure of ordinary ice. In the tetragonal structure, the water molecules are arranged in a more compact manner, resulting in a higher density.
5.2.3. Properties and Behavior
- Density: Ice III is denser than ordinary ice, with a density of approximately 1150 kg/m³.
- Stability: Ice III is stable only within a specific range of temperature and pressure. If the pressure is reduced or the temperature is increased, Ice III will revert back to ordinary ice or liquid water.
- Formation Mechanism: The formation of Ice III involves a rearrangement of the hydrogen bonds between water molecules. Under high pressure, the hydrogen bonds become distorted, and the water molecules shift to a more compact arrangement.
5.3. Equilibrium Between Ice Ih and Ice III
In a closed container, as water freezes under pressure, an equilibrium is eventually reached between Ice Ih (ordinary ice) and Ice III.
5.3.1. Pressure Increase
As water freezes, the pressure inside the container increases due to the expansion of the ice. This pressure increase can be significant, especially if the container is rigid and does not allow for much expansion.
5.3.2. Formation of Ice III
As the pressure increases, Ice III starts to form alongside Ice Ih. The proportion of Ice III increases with pressure until an equilibrium is reached.
5.3.3. Equilibrium Point
The equilibrium point depends on the temperature and the container’s ability to withstand pressure. At equilibrium, the rate of formation of Ice III equals the rate of its conversion back to Ice Ih or liquid water. The mixture of Ice Ih and Ice III results in a state where the pressure is balanced, and the system is stable.
5.3.4. Implications
This phenomenon has implications in various fields:
- Geophysics: Understanding the behavior of water under high pressure is crucial for studying the Earth’s interior, where water can exist in different phases.
- Material Science: Studying the behavior of materials under extreme conditions can lead to the development of new high-strength materials.
- Cryobiology: The formation of different ice phases can affect the preservation of biological samples at low temperatures.
Understanding the behavior of water under pressure and the different forms of ice that can result is essential for various scientific and technological applications.
6. Practical Implications: Why Floating Ice Matters
The fact that ice floats on water has profound implications for life on Earth, climate, and various industries.
6.1. Environmental Impact
6.1.1. Aquatic Life
One of the most significant environmental impacts of ice floating on water is its role in preserving aquatic life. When bodies of water such as lakes and oceans freeze, the ice forms at the surface. Because ice is less dense than liquid water, it remains on top, creating an insulating layer. This layer prevents the entire body of water from freezing solid, allowing fish and other aquatic organisms to survive the winter months.
The insulating effect of ice ensures that the temperature of the water beneath remains relatively stable, typically around 4°C (39°F), which is the temperature at which water is most dense. This stable environment allows aquatic life to continue their biological processes, such as feeding and respiration, albeit at a slower rate.
6.1.2. Climate Regulation
Ice and snow have a high albedo, which means they reflect a large proportion of the sunlight that reaches them back into space. This helps to regulate the Earth’s temperature by reducing the amount of solar radiation absorbed by the planet. The presence of ice cover, particularly in polar regions, plays a crucial role in maintaining global climate patterns.
The melting of ice due to climate change has a feedback effect: as ice melts, it exposes darker water or land surfaces, which absorb more sunlight. This leads to further warming, accelerating the melting process and contributing to rising sea levels.
6.1.3. Geological Processes
The expansion of water upon freezing is a powerful force that can cause significant geological changes. This phenomenon is known as ice wedging or frost weathering. When water seeps into cracks and crevices in rocks and then freezes, it expands by approximately 9%. This expansion exerts tremendous pressure on the surrounding rock, eventually causing it to fracture and break apart.
Ice wedging is particularly effective in mountainous and high-altitude regions where freeze-thaw cycles are common. Over time, this process contributes to the erosion of rocks and the formation of soil.
6.2. Industrial Applications
6.2.1. Ice Production and Storage
The unique properties of ice are utilized in various industrial applications, particularly in food preservation and cooling systems. The production of ice involves cooling water to its freezing point and then allowing it to solidify. The process often includes measures to control the formation of ice crystals to achieve the desired clarity and texture.
Ice is used extensively in the food industry to keep perishable items fresh during transportation and storage. It is also used in the beverage industry to chill drinks. The effectiveness of ice in these applications depends on its ability to absorb heat as it melts, providing a cooling effect.
6.2.2. Construction
In the construction industry, the expansion of water upon freezing can be both a problem and a solution. On one hand, it can cause damage to concrete structures if water seeps into pores and cracks and then freezes. To prevent this, air-entraining agents are added to concrete mixes to create tiny air bubbles that provide space for water to expand without causing structural damage.
On the other hand, the expansion of freezing water can be used in controlled demolition. By filling cracks in a structure with water and then freezing it, engineers can exert precise pressure to break apart the structure in a controlled manner.
6.2.3. Scientific Research
Ice is an important material in scientific research, particularly in fields such as glaciology, climate science, and materials science. Glaciologists study the properties and behavior of ice in glaciers and ice sheets to understand their role in the Earth’s climate system. Climate scientists use ice cores to reconstruct past climate conditions, as the ice contains trapped air bubbles and other materials that provide valuable information about the atmosphere at the time the ice was formed.
Materials scientists study the structure and properties of ice to develop new materials and technologies. For example, understanding the formation of ice crystals can lead to the development of improved methods for preserving biological tissues and organs at low temperatures.
| Application | Description | Benefit |
|---|---|---|
| Aquatic Life | Ice forms at the surface of water bodies, insulating the water below. | Allows aquatic organisms to survive winter by maintaining stable water temperatures. |
| Climate Regulation | Ice and snow reflect sunlight back into space. | Helps regulate Earth’s temperature and maintain global climate patterns. |
| Ice Wedging | Water expands when it freezes, breaking apart rocks. | Contributes to erosion and soil formation. |
| Food Preservation | Ice is used to keep perishable items fresh. | Extends shelf life and reduces spoilage. |
| Concrete | Air-entraining agents prevent damage from freezing water. | Prevents structural damage in concrete. |
| Climate Science | Ice cores provide data on past climate conditions. | Helps reconstruct past atmospheric conditions and understand climate change. |
7. Unveiling the Mysteries of Water: Further Exploration
The unique properties of water, including why ice floats, continue to be a subject of scientific inquiry and fascination. Here are some avenues for further exploration:
7.1. Research into Water’s Unusual Properties
7.1.1. Supercooled Water
Supercooled water is liquid water that is cooled below its freezing point (0°C or 32°F) without becoming solid. This phenomenon occurs because the water needs a nucleation site, such as an impurity or a rough surface, to start the ice crystal formation process. If the water is very pure and the container is smooth, the water molecules can remain in a liquid state at temperatures as low as -40°C (-40°F).
7.1.2. Quantum Mechanical Effects
Recent research suggests that quantum mechanical effects play a significant role in the behavior of water, particularly in the formation and breaking of hydrogen bonds. Quantum effects can influence the energy landscape of water molecules, affecting their interactions and leading to unique properties.
7.1.3. The Role of Isotopes
Water is composed of hydrogen and oxygen, both of which have different isotopes. Isotopes are atoms of the same element that have different numbers of neutrons. The presence of different isotopes in water can affect its properties, such as its density and freezing point.
7.2. Technological Applications Inspired by Ice
7.2.1. Ice-Based Cooling Systems
Ice-based cooling systems are used in various applications, such as air conditioning and food storage. These systems utilize the high latent heat of fusion of ice, which is the amount of energy required to melt ice without changing its temperature. Ice-based cooling systems can be more energy-efficient than traditional cooling systems, particularly in applications where cooling is needed intermittently.
7.2.2. Ice-Assisted Manufacturing
In manufacturing, ice can be used as a temporary support material or as a mold for creating complex shapes. For example, ice can be used to support thin-walled structures during the curing process. Once the structure is cured, the ice can be melted away, leaving the finished product.
7.2.3. Ice-Based Energy Storage
Ice can be used as a medium for storing thermal energy. During periods of low energy demand, excess electricity can be used to freeze water. The ice can then be stored until periods of high energy demand, at which point it can be melted to provide cooling.
7.3. Understanding Phase Transitions Under Extreme Conditions
7.3.1. High-Pressure Ice Phases
Under extreme pressures, water can form a variety of different ice phases, each with its unique crystal structure and properties. These high-pressure ice phases are of interest to scientists because they can provide insights into the behavior of matter under extreme conditions.
7.3.2. Superionic Ice
Superionic ice is a phase of water that exists at extremely high temperatures and pressures. In superionic ice, the oxygen atoms form a crystal lattice, while the hydrogen ions move freely through the lattice. This phase of water is thought to exist in the interiors of large planets, such as Uranus and Neptune.
7.3.3. The Glass Transition of Water
When water is cooled rapidly, it can bypass the crystalline state and form a glassy state, also known as amorphous ice. The glass transition of water is a complex phenomenon that is still not fully understood.
8. FAQ: Addressing Common Questions About Ice and Water
Here are some frequently asked questions to further clarify the topic of why ice floats on water:
-
Why is water so unique compared to other substances?
Water’s uniqueness stems from its polar nature and hydrogen bonding, leading to high surface tension, unusual density behavior, and excellent solvent properties.
-
Does all ice float, or are there exceptions?
Typically, ice made from pure water floats. However, ice formed from saltwater can sometimes be denser due to the incorporation of salt ions.
-
How does the temperature of water affect its density?
Water reaches maximum density at 4°C. Above and below this temperature, its density decreases.
-
What role does pressure play in the different phases of ice?
Pressure influences the formation of different ice phases. At high pressures, water can form denser ice structures.
-
Can we create ice that sinks in water?
Yes, by increasing the pressure on water while freezing it, you can create denser forms of ice that sink.
-
What are the practical implications of ice floating on water for marine ecosystems?
Floating ice insulates water bodies, preventing them from freezing entirely and allowing aquatic life to survive.
-
How does climate change impact the phenomenon of floating ice?
Climate change leads to melting ice, reducing Earth’s albedo and causing further warming, disrupting ecosystems and weather patterns.
-
What is the role of hydrogen bonds in the density of ice?
Hydrogen bonds cause water molecules to arrange in an open lattice structure when frozen, making ice less dense than liquid water.
-
Why does ice expand when it freezes?
The hydrogen bonds in ice create a crystalline structure that takes up more space than the liquid state, causing expansion.
-
Are there any other substances that behave like water in terms of density?
Water’s density anomaly is rare. Bismuth and gallium also expand upon freezing, but the effect is much less pronounced.
9. Discover More at WHY.EDU.VN
Exploring the question “Why can ice float on water?” reveals fundamental properties of water and their significance in our world. From buoyancy principles to the unique molecular structure of water, many factors contribute to this phenomenon.
Do you have more questions about science, nature, or any other topic? Visit WHY.EDU.VN at 101 Curiosity Lane, Answer Town, CA 90210, United States, or contact us via WhatsApp at +1 (213) 555-0101. At why.edu.vn, we provide comprehensive answers and expert insights to satisfy your curiosity. Dive into our wealth of knowledge and unlock the answers to your most pressing questions today. We are dedicated to delivering accurate, reliable, and engaging content. Visit us today.