Why Water Is Called A Universal Solvent? Water’s exceptional ability to dissolve a wide array of substances earns it the title of universal solvent, a crucial attribute that supports life as we know it. This remarkable property stems from water’s unique molecular structure and polarity, allowing it to transport vital chemicals, minerals, and nutrients throughout the environment and within living organisms, as highlighted by why.edu.vn. Discover how this impacts our health, environment, and various industries, revealing the profound importance of water solubility.
1. Understanding Water’s Universal Solvent Property
Water’s designation as the “universal solvent” stems from its remarkable capability to dissolve more substances than any other known liquid. This property is critical for a myriad of biological, environmental, and industrial processes. Water’s effectiveness as a solvent arises from its molecular structure and polarity, which enable it to interact with and dissolve a wide range of compounds.
1.1. Defining a Solvent
A solvent is a substance that dissolves a solute (a solid, liquid, or gas) to form a solution. The solvent essentially acts as the medium that disperses the solute molecules, resulting in a homogeneous mixture. The ability of a solvent to dissolve different substances depends on its chemical properties and the nature of the solute.
1.2. What Makes Water a Universal Solvent?
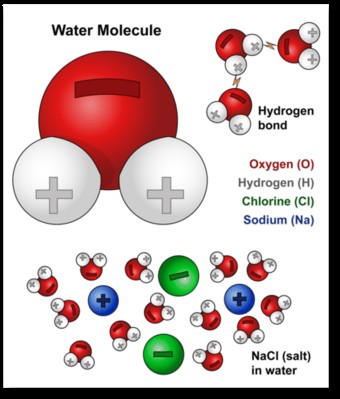
Water’s solvent properties are primarily due to its polar nature. A water molecule consists of one oxygen atom and two hydrogen atoms (H2O). Oxygen is more electronegative than hydrogen, meaning it attracts electrons more strongly, leading to a partial negative charge (δ-) on the oxygen atom and partial positive charges (δ+) on the hydrogen atoms. This creates a dipole moment within the molecule, making water polar.
The polarity of water enables it to interact with other polar molecules and ionic compounds. When water comes into contact with a polar substance, the positive end of the water molecule attracts the negative end of the solute molecule, and vice versa. This interaction weakens the intermolecular forces holding the solute together, allowing it to disperse evenly throughout the water.
 Water Molecule Polarity
Water Molecule Polarity
1.3. Polarity Explained
To elaborate on polarity, consider the electronegativity difference between oxygen and hydrogen in a water molecule. Electronegativity is a measure of an atom’s ability to attract electrons in a chemical bond. Oxygen has a higher electronegativity (3.44 on the Pauling scale) than hydrogen (2.20). This results in the shared electrons in the O-H bonds being drawn closer to the oxygen atom, giving it a partial negative charge and leaving the hydrogen atoms with partial positive charges.
This uneven distribution of charge creates a dipole moment, which is a measure of the separation of positive and negative charges in a molecule. The dipole moment of water (1.85 Debye units) is relatively high compared to other molecules, indicating its strong polarity.
1.4. Hydrogen Bonding and Solvency
The polarity of water also leads to the formation of hydrogen bonds. Hydrogen bonds are relatively weak intermolecular forces that occur between a hydrogen atom bonded to a highly electronegative atom (such as oxygen) and another electronegative atom in a different molecule. In water, hydrogen bonds form between the partially positive hydrogen atoms of one water molecule and the partially negative oxygen atoms of another.
These hydrogen bonds contribute to water’s high surface tension, cohesion, and adhesion properties, as well as its solvent capabilities. The hydrogen bonds help to stabilize the interactions between water molecules and solute particles, facilitating the dissolution process.
1.5. Dissolving Ionic Compounds
Water is particularly effective at dissolving ionic compounds like sodium chloride (NaCl), common table salt. When salt is added to water, the positive sodium ions (Na+) are attracted to the partially negative oxygen atoms of water molecules, while the negative chloride ions (Cl-) are attracted to the partially positive hydrogen atoms.
This attraction weakens the ionic bonds holding the sodium and chloride ions together in the salt crystal. Water molecules surround each ion, effectively isolating it from the crystal lattice. This process is called solvation or hydration. The hydrated ions are then dispersed throughout the water, resulting in the dissolution of the salt.
1.6. Dissolving Polar Covalent Compounds
Water can also dissolve polar covalent compounds such as ethanol (C2H5OH) and sucrose (C12H22O11). Ethanol, found in alcoholic beverages, has a hydroxyl group (-OH) that makes it polar. The oxygen atom in the hydroxyl group attracts electrons, creating a partial negative charge, while the hydrogen atom has a partial positive charge. This allows ethanol to form hydrogen bonds with water molecules, facilitating its dissolution.
Sucrose, or table sugar, is a larger polar molecule with multiple hydroxyl groups. These hydroxyl groups can form hydrogen bonds with water molecules, allowing sucrose to dissolve in water. The more hydroxyl groups a molecule has, the more soluble it tends to be in water.
1.7. Limitations of Water as a Solvent
Despite its versatility, water is not a universal solvent in the strictest sense. It does not dissolve all substances. Nonpolar substances, such as oils and fats, do not dissolve well in water. These substances are composed of molecules with an even distribution of charge, so they do not interact strongly with polar water molecules.
When oil and water are mixed, the nonpolar oil molecules tend to clump together to minimize their contact with water. This is because the water molecules are more attracted to each other through hydrogen bonds than they are to the nonpolar oil molecules. This results in the formation of separate layers, with the less dense oil floating on top of the water.
1.8. Other Solvents
Other solvents exist that are better suited for dissolving nonpolar substances. These include organic solvents like hexane, toluene, and chloroform. These solvents are nonpolar themselves, so they can interact with and dissolve nonpolar solutes more effectively than water can.
Different solvents are used in various industrial and laboratory applications depending on the specific substances that need to be dissolved. Water remains the most widely used solvent due to its abundance, low cost, and nontoxic nature, making it ideal for many applications in biology, chemistry, and environmental science.
1.9. Temperature and Pressure Effects on Solubility
Temperature and pressure can also affect the solubility of substances in water. Generally, the solubility of solid solutes in water increases with temperature. This is because higher temperatures provide more kinetic energy to the solute molecules, making it easier for them to overcome the intermolecular forces holding them together and dissolve in the water.
For example, more sugar can be dissolved in hot water than in cold water. The increased temperature allows the water molecules to break apart the sucrose molecules more effectively, leading to a higher concentration of dissolved sugar.
The effect of temperature on the solubility of gases in water is the opposite. The solubility of gases decreases with increasing temperature. This is because higher temperatures increase the kinetic energy of the gas molecules, making them more likely to escape from the liquid.
Pressure also affects the solubility of gases in water. According to Henry’s Law, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. This means that increasing the pressure of a gas above water will increase the amount of gas that dissolves in the water.
1.10. Environmental Significance of Water as a Solvent
Water’s solvent properties play a crucial role in the environment. Water transports nutrients and minerals in soil to plants, carries dissolved salts and minerals in rivers and oceans, and facilitates chemical reactions in aquatic ecosystems.
In soil, water dissolves essential nutrients such as nitrogen, phosphorus, and potassium, making them available for plants to absorb through their roots. Without water’s solvent action, plants would not be able to obtain the nutrients they need to grow and survive.
In aquatic environments, water dissolves salts, minerals, and organic compounds, creating a complex chemical environment that supports a wide variety of organisms. The salinity of seawater, which is primarily due to dissolved sodium chloride, affects the density, buoyancy, and freezing point of water, influencing ocean currents and marine life.
1.11. Biological Significance of Water as a Solvent
Water’s solvent properties are essential for life. It facilitates the transport of nutrients and waste products in organisms, enables biochemical reactions within cells, and helps to regulate body temperature.
In animals, blood is primarily composed of water, which transports oxygen, nutrients, hormones, and other essential substances to cells throughout the body. Water also carries waste products, such as carbon dioxide and urea, away from cells to be excreted.
Within cells, water acts as a solvent for many biochemical reactions. Enzymes, which are biological catalysts, require water to function properly. Water also participates directly in many metabolic reactions, such as hydrolysis, where water is used to break down complex molecules into smaller units.
Water’s high heat capacity and thermal conductivity also help to regulate body temperature. Water can absorb a large amount of heat without undergoing a significant temperature change, which helps to prevent overheating. Sweating, which is the evaporation of water from the skin, is an effective way to cool the body.
1.12. Industrial Applications of Water as a Solvent
Water is widely used as a solvent in various industrial processes. It is used in the production of chemicals, pharmaceuticals, food products, and many other materials.
In the chemical industry, water is used as a solvent for chemical reactions, extraction, and purification processes. It is also used as a coolant to regulate temperature in reactors and other equipment.
In the pharmaceutical industry, water is used as a solvent for drug formulations, cleaning equipment, and sterilization processes. Water must be highly purified to meet the stringent quality standards required for pharmaceutical products.
In the food industry, water is used for washing, cooking, and processing food products. It is also used as a solvent for extracting flavors, colors, and other ingredients from raw materials.
1.13. Water Quality and Solvent Properties
The quality of water can affect its solvent properties. Impurities in water, such as dissolved salts, organic matter, and pollutants, can alter its ability to dissolve other substances.
Hard water, which contains high concentrations of calcium and magnesium ions, can interfere with the action of soaps and detergents, reducing their effectiveness. Pollutants, such as heavy metals and organic chemicals, can also affect water’s solvent properties and pose risks to human health and the environment.
Water treatment processes, such as filtration, distillation, and reverse osmosis, can be used to remove impurities and improve water quality. Purified water is often required for laboratory experiments, industrial processes, and pharmaceutical applications.
1.14. Future Research and Applications
Research continues to explore new applications of water’s solvent properties. Scientists are investigating the use of water as a green solvent for chemical reactions, reducing the reliance on harmful organic solvents. Water is also being studied as a medium for drug delivery, enhancing the bioavailability and effectiveness of medications.
Advances in nanotechnology are creating new opportunities to manipulate water’s solvent properties at the molecular level. Researchers are developing new materials and techniques to control the interactions between water and other substances, with potential applications in areas such as water purification, energy storage, and materials science.
Water’s role as a universal solvent is fundamental to life and technology. Understanding its properties and limitations is essential for addressing challenges in areas such as environmental protection, human health, and industrial development. With ongoing research and innovation, water’s solvent capabilities will continue to be harnessed for the benefit of society.
2. The Chemical Composition of Water
Water is composed of two hydrogen atoms and one oxygen atom, forming the molecule H2O. This simple structure belies its complex properties, which are fundamental to its role as a universal solvent.
2.1. Molecular Structure of Water
The water molecule has a bent shape, with an angle of approximately 104.5 degrees between the two hydrogen atoms. This bent geometry is due to the electron repulsion between the two bonding pairs and the two lone pairs of electrons on the oxygen atom.
The oxygen atom is more electronegative than the hydrogen atoms, meaning it attracts electrons more strongly. This results in an uneven distribution of charge within the molecule, with the oxygen atom carrying a partial negative charge (δ-) and the hydrogen atoms carrying partial positive charges (δ+). This charge separation creates a dipole moment, making the water molecule polar.
2.2. Hydrogen Bonding in Water
The polarity of water molecules leads to the formation of hydrogen bonds. A hydrogen bond is a relatively weak interaction between a partially positive hydrogen atom in one molecule and a partially negative atom (such as oxygen) in another molecule.
In water, hydrogen bonds form between the partially positive hydrogen atoms of one water molecule and the partially negative oxygen atoms of another. Each water molecule can form up to four hydrogen bonds with neighboring water molecules, creating a dynamic network of interconnected molecules.
2.3. Properties Influenced by Hydrogen Bonding
Hydrogen bonding gives water its unique properties, including high surface tension, high boiling point, and high heat capacity.
2.3.1. Surface Tension
Surface tension is the tendency of the surface of a liquid to minimize its area. Water has a high surface tension due to the cohesive forces between water molecules at the surface. These molecules are pulled inward by hydrogen bonds with other water molecules below, creating a tight surface layer.
2.3.2. Boiling Point
The boiling point of a liquid is the temperature at which it changes from a liquid to a gas. Water has a relatively high boiling point (100°C or 212°F) compared to other liquids of similar molecular weight. This is because the hydrogen bonds between water molecules must be broken before they can escape into the gaseous phase.
2.3.3. Heat Capacity
Heat capacity is the amount of heat required to raise the temperature of a substance by a certain amount. Water has a high heat capacity, meaning it can absorb a large amount of heat without undergoing a significant temperature change. This is due to the energy required to break and form hydrogen bonds as the temperature increases.
2.4. Impact on Solvent Properties
The chemical composition and structure of water, including its polarity and hydrogen bonding, directly influence its solvent properties. Water’s ability to dissolve a wide range of substances is primarily due to its polar nature, which allows it to interact with and solvate both ionic and polar compounds.
Water’s hydrogen bonding also plays a role in its solvent capabilities. Hydrogen bonds help to stabilize the interactions between water molecules and solute particles, facilitating the dissolution process.
2.5. Hydrophilic and Hydrophobic Substances
Substances that dissolve readily in water are called hydrophilic, meaning “water-loving.” These substances are typically polar or ionic and can form hydrogen bonds with water molecules. Examples of hydrophilic substances include salts, sugars, and alcohols.
Substances that do not dissolve well in water are called hydrophobic, meaning “water-fearing.” These substances are typically nonpolar and cannot form hydrogen bonds with water molecules. Examples of hydrophobic substances include oils, fats, and waxes.
2.6. Amphipathic Molecules
Some molecules have both hydrophilic and hydrophobic regions. These molecules are called amphipathic. Soaps and detergents are examples of amphipathic molecules. They have a polar head that is attracted to water and a nonpolar tail that is attracted to oils and fats.
Amphipathic molecules can form micelles in water, which are spherical aggregates with the nonpolar tails pointing inward and the polar heads pointing outward. Micelles can dissolve nonpolar substances in their interior, allowing them to be washed away by water.
2.7. Hydration Shells
When an ionic compound dissolves in water, each ion is surrounded by a shell of water molecules. This shell is called a hydration shell. The water molecules in the hydration shell are oriented with their partially charged ends pointing toward the ion, with the positive ends oriented toward negative ions and the negative ends oriented toward positive ions.
The hydration shell helps to stabilize the ions in solution and prevent them from recombining. It also reduces the attraction between ions of opposite charge, allowing them to remain dissolved.
2.8. Water as a Reaction Medium
Water is not only a solvent but also a reaction medium. Many chemical reactions occur in water, including acid-base reactions, oxidation-reduction reactions, and hydrolysis reactions.
Water can act as both an acid and a base, donating or accepting protons (H+) in chemical reactions. It also participates directly in hydrolysis reactions, where water is used to break down complex molecules into smaller units.
2.9. Water in Biological Systems
Water plays a critical role in biological systems. It is the primary component of cells, tissues, and organs and is essential for many biological processes, including photosynthesis, respiration, and digestion.
Water transports nutrients and waste products in organisms, facilitates biochemical reactions within cells, and helps to regulate body temperature. Its unique properties make it an ideal medium for life.
2.10. The Importance of Clean Water
The quality of water is crucial for both human health and the environment. Polluted water can contain harmful chemicals, pathogens, and other contaminants that can cause disease and damage ecosystems.
Access to clean, safe water is a fundamental human right. Water treatment processes, such as filtration, disinfection, and desalination, are used to remove impurities and ensure that water is safe to drink and use.
2.11. The Future of Water Research
Research continues to explore the unique properties of water and its role in various processes. Scientists are investigating the structure of water at the molecular level, developing new methods for water purification, and studying the impact of climate change on water resources.
Understanding water’s chemical composition and properties is essential for addressing challenges in areas such as environmental sustainability, human health, and industrial development. With ongoing research and innovation, we can continue to harness water’s capabilities for the benefit of society.
3. Physical Attributes Contributing to Water’s Solvent Ability
Besides its chemical composition, several physical attributes of water contribute to its exceptional solvent capabilities. These include its polarity, hydrogen bonding, density, and thermal properties.
3.1. Polarity and Dipole Moment
As previously discussed, water’s polarity is a critical factor in its solvent ability. The bent shape of the water molecule and the electronegativity difference between oxygen and hydrogen create a dipole moment, resulting in a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms.
This polarity allows water molecules to interact with and dissolve ionic and polar compounds. The positive end of the water molecule attracts negative ions or the negative end of polar molecules, while the negative end of the water molecule attracts positive ions or the positive end of polar molecules.
3.2. Hydrogen Bonding and Cohesion
Hydrogen bonding between water molecules is another important physical attribute that contributes to its solvent ability. Each water molecule can form up to four hydrogen bonds with neighboring water molecules, creating a dynamic network of interconnected molecules.
These hydrogen bonds give water its high surface tension, high boiling point, and high heat capacity. They also contribute to water’s cohesion, which is the attraction between water molecules themselves. Cohesion helps to stabilize the interactions between water molecules and solute particles, facilitating the dissolution process.
3.3. Density and Buoyancy
Water’s density is also important for its role as a solvent. Water is most dense at 4°C (39°F), which is why ice floats on water. This is because the hydrogen bonds in ice form a crystalline structure that is less dense than liquid water.
The fact that ice floats on water is crucial for aquatic life. It insulates the water below, preventing it from freezing solid and allowing organisms to survive the winter. Water’s density also affects buoyancy, which is the ability of a substance to float in water. Substances that are less dense than water will float, while substances that are more dense will sink.
3.4. Thermal Properties
Water’s thermal properties, including its high heat capacity and high heat of vaporization, are also important for its role as a solvent. Water’s high heat capacity allows it to absorb a large amount of heat without undergoing a significant temperature change. This helps to regulate temperature in organisms and in the environment.
Water’s high heat of vaporization, which is the amount of heat required to convert a liquid to a gas, makes evaporative cooling an effective way to regulate body temperature. Sweating, which is the evaporation of water from the skin, cools the body by removing heat.
3.5. Dielectric Constant
Water has a high dielectric constant, which is a measure of its ability to reduce the electrostatic attraction between oppositely charged ions. A high dielectric constant means that water can effectively shield ions from each other, allowing them to remain dissolved in solution.
The dielectric constant of water is about 80 at room temperature, which is much higher than that of most other solvents. This is due to water’s polarity and its ability to form hydrogen bonds.
3.6. Viscosity
Water has a relatively low viscosity, which is a measure of its resistance to flow. Low viscosity means that water can easily flow through small spaces, such as pores in soil or capillaries in blood vessels. This allows water to transport nutrients and waste products throughout organisms and in the environment.
Water’s viscosity is affected by temperature. As temperature increases, water’s viscosity decreases, making it easier for water to flow.
3.7. Surface Tension and Capillary Action
Water’s high surface tension, as discussed earlier, contributes to capillary action, which is the ability of water to move up narrow tubes or spaces against the force of gravity. Capillary action is important for the transport of water in plants and in soil.
Water is drawn up through the xylem of plants by capillary action, allowing it to reach the leaves where it is used for photosynthesis. Water also moves through the pores in soil by capillary action, providing moisture to plant roots.
3.8. Transparency
Water is transparent to visible light, which allows sunlight to penetrate into aquatic environments. This is essential for photosynthesis, which is the process by which plants and algae convert sunlight into chemical energy.
The depth to which sunlight can penetrate into water depends on its clarity. Clear water allows sunlight to penetrate deeper than murky water.
3.9. Solvent Properties and Phase Changes
Water’s solvent properties are affected by its phase (solid, liquid, or gas). Water is most effective as a solvent in its liquid phase. In the solid phase (ice), the crystalline structure limits the movement of water molecules and reduces their ability to interact with solute particles. In the gas phase (steam), water molecules are too far apart to effectively solvate other substances.
The phase changes of water (melting, freezing, boiling, condensation) involve significant changes in energy and volume. These changes can affect water’s solvent properties and its role in various processes.
3.10. Water and Mineral Solubility
The solubility of minerals in water is affected by several factors, including temperature, pressure, pH, and the presence of other ions. Generally, the solubility of most minerals increases with increasing temperature and decreasing pH.
Water plays a crucial role in the weathering of rocks and the formation of soil. It dissolves minerals from rocks, transporting them to other locations where they can precipitate and form new minerals.
3.11. Water and Organic Matter
Water also interacts with organic matter in the environment. It dissolves organic compounds, transports them, and participates in their decomposition.
The amount of organic matter in water can affect its quality. High levels of organic matter can lead to oxygen depletion, which can harm aquatic life.
3.12. Water and Colloids
Colloids are mixtures that contain particles that are larger than molecules but smaller than particles that can be seen with the naked eye. Water can act as a solvent for colloids, keeping the particles dispersed in solution.
Colloids are common in many natural and industrial systems. Milk, paint, and fog are examples of colloids.
3.13. The Importance of Water Purification
The purity of water is essential for many applications. Impurities in water can affect its solvent properties and its suitability for various uses.
Water purification processes, such as filtration, distillation, and reverse osmosis, are used to remove impurities and ensure that water is safe to drink and use.
3.14. Future Directions in Water Research
Research continues to explore the physical attributes of water and their impact on its solvent properties. Scientists are investigating the structure of water at the nanoscale, developing new methods for water purification, and studying the role of water in climate change.
Understanding water’s physical attributes is essential for addressing challenges in areas such as environmental sustainability, human health, and industrial development. With ongoing research and innovation, we can continue to harness water’s capabilities for the benefit of society.
4. The Role of Water in Biological Systems
Water is indispensable for life, serving as the primary solvent within biological systems. Its unique properties facilitate numerous biological processes, from nutrient transport to waste removal.
4.1. Water as a Transport Medium
In living organisms, water acts as a transport medium, carrying nutrients, gases, and waste products to and from cells. Blood, which is primarily composed of water, transports oxygen from the lungs to the tissues and carbon dioxide from the tissues to the lungs.
Water also transports nutrients from the digestive system to the cells and waste products from the cells to the excretory organs. Its solvent properties allow it to dissolve a wide range of substances, making it an ideal transport medium.
4.2. Water in Cellular Processes
Water is involved in many cellular processes, including metabolism, photosynthesis, and respiration. It acts as a solvent for biochemical reactions, a reactant in hydrolysis reactions, and a product in dehydration reactions.
Enzymes, which are biological catalysts, require water to function properly. Water also helps to maintain the structure and function of proteins, nucleic acids, and other biomolecules.
4.3. Water and Temperature Regulation
Water’s high heat capacity and high heat of vaporization make it an excellent temperature regulator. It can absorb a large amount of heat without undergoing a significant temperature change, helping to prevent overheating.
Sweating, which is the evaporation of water from the skin, is an effective way to cool the body. The evaporation of water removes heat from the skin, lowering body temperature.
4.4. Water in Plant Physiology
In plants, water is essential for photosynthesis, nutrient transport, and structural support. It is a reactant in photosynthesis, providing the electrons needed to convert carbon dioxide into glucose.
Water also transports nutrients from the soil to the leaves and provides turgor pressure, which helps to keep plant cells rigid and maintain their shape.
4.5. Water and Waste Removal
Water is involved in the removal of waste products from the body. The kidneys, which are the primary organs of excretion, filter waste products from the blood and excrete them in urine.
Sweat, which is composed of water and dissolved salts, also helps to remove waste products from the body.
4.6. Water and Digestive Processes
Water is essential for digestive processes. It is a component of saliva, which helps to moisten food and begin the process of digestion.
Water also participates in hydrolysis reactions, which break down complex molecules into smaller units that can be absorbed by the body.
4.7. Water and Lubrication
Water acts as a lubricant in the body, reducing friction between joints and organs. Synovial fluid, which lubricates joints, is primarily composed of water.
Mucus, which lines the respiratory and digestive tracts, also contains water and helps to protect these surfaces from damage.
4.8. Water and Sensory Perception
Water is involved in sensory perception. The eyes, which are responsible for vision, contain a clear fluid called the aqueous humor, which is primarily composed of water.
The ears, which are responsible for hearing, contain a fluid called endolymph, which is also primarily composed of water.
4.9. Water and Reproduction
Water is essential for reproduction. Semen, which is the fluid that carries sperm, contains water.
Amniotic fluid, which surrounds the developing fetus in the uterus, is also primarily composed of water.
4.10. Water and Immune Function
Water is involved in immune function. Lymph, which is a fluid that circulates throughout the body, contains water and helps to transport immune cells to sites of infection.
Tears, which are produced by the lacrimal glands, contain water and help to protect the eyes from infection.
4.11. Dehydration and Its Effects
Dehydration, which is a deficiency of water in the body, can have serious health consequences. Symptoms of dehydration include thirst, fatigue, headache, dizziness, and decreased urine output.
Severe dehydration can lead to organ damage, coma, and death.
4.12. Maintaining Hydration
Maintaining proper hydration is essential for health. The amount of water that a person needs each day depends on factors such as activity level, climate, and overall health.
The Institute of Medicine recommends that men consume about 3.7 liters (15.5 cups) of water per day and women consume about 2.7 liters (11.5 cups) of water per day.
4.13. Water and Exercise
Exercise increases the need for water. During exercise, the body loses water through sweat, which can lead to dehydration.
Athletes should drink plenty of water before, during, and after exercise to maintain proper hydration.
4.14. Water and Aging
The need for water may decrease with age due to reduced kidney function and decreased thirst sensation. However, older adults are still at risk of dehydration and should make an effort to drink enough water each day.
4.15. The Importance of Clean Drinking Water
Access to clean drinking water is essential for health. Contaminated water can contain harmful bacteria, viruses, and parasites that can cause disease.
Water treatment processes, such as filtration, disinfection, and desalination, are used to remove contaminants and ensure that water is safe to drink.
4.16. Future Directions in Biological Water Research
Research continues to explore the role of water in biological systems. Scientists are investigating the structure of water within cells, developing new methods for water purification, and studying the effects of dehydration on health.
Understanding water’s role in biological systems is essential for addressing challenges in areas such as human health, disease prevention, and environmental sustainability. With ongoing research and innovation, we can continue to harness water’s capabilities for the benefit of society.
5. The Significance of Water’s Solvent Properties in Daily Life
Water’s solvent properties are not just a scientific curiosity; they have profound implications for our daily lives, influencing everything from cooking to cleaning and beyond.
5.1. Cooking and Food Preparation
Water is an essential ingredient in cooking and food preparation. It is used to dissolve ingredients, such as salt, sugar, and spices, and to cook foods, such as pasta, rice, and vegetables.
Water also helps to extract flavors from ingredients, such as tea leaves and coffee beans.
5.2. Cleaning and Sanitation
Water is used for cleaning and sanitation. It dissolves dirt, grime, and other contaminants, allowing them to be washed away.
Soaps and detergents, which are amphipathic molecules, help to emulsify oils and fats, making them easier to remove with water.
5.3. Laundry and Fabric Care
Water is used for laundry and fabric care. It dissolves dirt, stains, and odors from clothing and other textiles.
Laundry detergents, which contain surfactants and enzymes, help to remove stubborn stains and brighten colors.
5.4. Personal Hygiene
Water is used for personal hygiene. It helps to remove dirt, sweat, and bacteria from the skin and hair.
Soaps and shampoos, which contain detergents, help to remove oils and grime, leaving the skin and hair clean and fresh.
5.5. Gardening and Agriculture
Water is essential for gardening and agriculture. It provides moisture to plant roots, transports nutrients, and helps to regulate plant temperature.
Irrigation systems are used to supply water to crops in areas with insufficient rainfall.
5.6. Industrial Processes
Water is used in many industrial processes. It is used as a solvent, a coolant, a cleaning agent, and a reactant.
The chemical, pharmaceutical, and food industries all rely heavily on water.
5.7. Water Treatment and Purification
Water treatment and purification are essential for ensuring that water is safe to drink and use. Treatment processes, such as filtration, disinfection, and desalination, remove contaminants from water, making it suitable for various purposes.
5.8. Water and Energy Production
Water is used in energy production. It is used to cool power plants, generate steam, and produce hydroelectric power.
5.9. Water and Transportation
Water is used for transportation. Ships and boats use water to navigate oceans, rivers, and lakes.
Waterways provide a cost-effective means of transporting goods and people.
5.10. Water and Recreation
Water is used for recreation. Swimming, boating, fishing, and water skiing are all popular recreational activities.
5.11. Water and Art
Water is used in art. It is used as a solvent for paints, inks, and dyes.
Watercolors, which are paints made with water-soluble pigments, are a popular medium for artists.
5.12. Water and Spirituality
Water is often associated with spirituality. It is used in religious ceremonies, such as baptisms, and is considered a symbol of purity and cleansing.
5.13. Water and Health
Water is essential for health. Drinking enough water helps to maintain proper hydration, regulate body temperature, and remove waste products.
Dehydration can lead to serious health problems, so it is important to drink plenty of water each day.
5.14. Water and Environmental Sustainability
Water is essential for environmental sustainability. Conserving water, reducing pollution, and protecting watersheds are all important for ensuring that future generations have access to clean, safe water.
5.15. Water and Climate Change
Climate change is affecting water resources around the world. Rising temperatures, changing precipitation patterns, and increased frequency of extreme weather events are all impacting water availability and quality.
5.16. Future Directions in Water Usage
Research continues to explore new ways to use water more efficiently and sustainably. Scientists are developing new technologies for water purification, irrigation, and wastewater treatment.
Understanding water’s solvent properties is essential for addressing challenges in areas such as human health, environmental sustainability, and industrial development. With ongoing research and innovation, we can continue to harness water’s capabilities for the benefit of society.
Water’s exceptional solvency makes it an indispensable component of countless aspects of our lives, from sustaining life itself to enabling a vast array of industrial and technological advancements.
6. Common Misconceptions About Water as a Solvent
Despite its well-known solvent capabilities, several misconceptions exist regarding water’s properties and limitations. Addressing these misconceptions can lead to a better understanding of water’s role in various processes.
6.1. Water Dissolves Everything
A common misconception is that water can dissolve any substance. While water is an excellent solvent for many ionic and polar compounds, it does not dissolve nonpolar substances, such as oils and fats.
The term “universal solvent” is often used to describe water, but it is important to remember that this is an idealization rather than an absolute truth.
6.2. Pure Water is a Good Conductor of Electricity
Another misconception is that pure water is a good conductor of electricity. In reality, pure water is a poor conductor of electricity. It is the presence of dissolved ions, such as salts and minerals, that makes water conductive.
Deionized water, which is water that has had most of its ions removed, is a very poor conductor of electricity.
6.3. Water is Always Safe to Drink
A common misconception is that water is always safe to drink. Untreated water can contain harmful bacteria, viruses, and parasites that can cause disease.
Water treatment processes, such as filtration, disinfection, and desalination, are used to remove contaminants and ensure that water is safe to drink.
6.4. Boiling Water Removes All Impurities
Boiling water can kill many harmful bacteria and viruses, but it does not remove all impurities. It does not remove dissolved salts, minerals, or heavy metals.
Distillation is a more effective method for removing impurities from water.
6.5. Bottled Water is Always Purer Than Tap Water
Bottled water is not always purer than tap water. In some cases, bottled water may be less pure than tap water.
The quality of bottled water depends on the source of the water and the treatment processes used.
6.6. All Water Filters are Equally Effective
All water filters are not equally effective. Different types of filters remove different types of contaminants.
Some filters are designed to remove sediment and chlorine, while others are designed to remove heavy metals and bacteria.
6.7. Soft Water is Healthier Than Hard Water
Soft water is not necessarily healthier than hard water. Soft water contains fewer minerals, such as calcium and magnesium, than hard water.
Some people prefer the taste of hard water, while others prefer the taste of soft water.
6.8. Desalination is a Perfect Solution for Water Scarcity
Desalination, which is the process of removing salt from seawater, is not a perfect solution for water scarcity. It is an expensive process that requires a lot of energy.
Desalination can also have negative environmental impacts, such as the release of concentrated brine into the ocean.
6.9. Water Conservation is Only Important in Dry Areas
Water conservation is not only important in dry areas. Water is a limited resource, and it is important to use it wisely, regardless of where you live.
Cons
