Water’s remarkable ability to dissolve a vast array of substances earns it the title of “universal solvent.” This property is crucial for life on Earth, impacting everything from nutrient transport in ecosystems to vital bodily functions. But what makes water such an exceptional solvent? Let’s delve into the chemistry behind this phenomenon.
The Polarity of Water: The Key to Its Dissolving Power
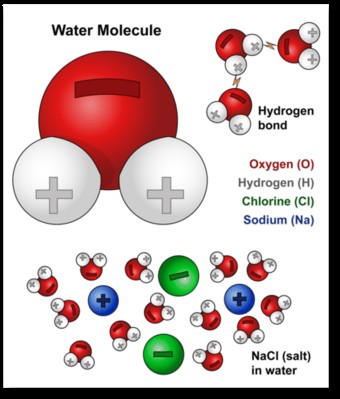
Water’s exceptional solvent properties stem from its unique molecular structure. A water molecule (H2O) consists of two hydrogen atoms and one oxygen atom. The arrangement of these atoms creates a polar molecule, meaning it has a slightly positive end (hydrogen side) and a slightly negative end (oxygen side).
This polarity allows water molecules to interact with other polar molecules and ions. Like tiny magnets, the positive end of a water molecule attracts negative particles, while the negative end attracts positive particles. This attraction is what enables water to dissolve a wide range of substances, from salts and sugars to minerals and even some gases.
The Dissolving Process: A Tug-of-War Between Molecules
When a substance dissolves in water, the water molecules surround and separate the individual particles of that substance. For example, when salt (sodium chloride, NaCl) is added to water, the positive sodium ions (Na+) are attracted to the negative oxygen ends of water molecules, while the negative chloride ions (Cl-) are attracted to the positive hydrogen ends.
This attraction is stronger than the forces holding the sodium and chloride ions together in the salt crystal. As a result, the water molecules pull the ions apart, effectively dissolving the salt. The separated ions become surrounded by water molecules, forming a homogeneous solution. This process, illustrated below, showcases how the polar nature of water disrupts the ionic bonds in salt.
Water as a Universal Solvent: Importance for Life
Water’s role as a universal solvent is vital for life as we know it. In biological systems, water dissolves essential nutrients and minerals, allowing them to be transported throughout organisms. It also helps remove waste products, keeping cells and tissues functioning properly.
For instance, our kidneys rely on water’s solvent properties to filter waste products from the blood. Water dissolves these waste products, allowing them to be excreted from the body in urine. Without water’s ability to dissolve and transport substances, life as we know it would be impossible.
Beyond Salts: Water’s Versatile Dissolving Abilities
While water dissolves many ionic compounds like salt exceptionally well, its versatility extends further. It can also dissolve polar molecules like sugars and even some nonpolar molecules to a lesser extent. This broad dissolving power makes water a crucial component in numerous natural and industrial processes.
In conclusion, water’s designation as the “universal solvent” is well-deserved. Its polar nature allows it to dissolve a wider variety of substances than any other liquid, playing a fundamental role in supporting life and driving various chemical and biological processes on Earth.