Water’s remarkable ability to dissolve various substances stems from its unique polar nature. But what exactly does it mean for water to be polar? This characteristic arises from the specific arrangement of its atoms and the resulting distribution of electrical charges. Understanding water’s polarity is crucial to grasping its role as a universal solvent and its importance in various biological and chemical processes.
The Polarity of Water Molecules
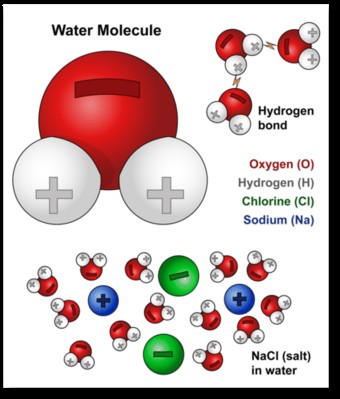
A water molecule (H2O) consists of two hydrogen atoms and one oxygen atom. These atoms are connected by covalent bonds, where they share electrons. However, the sharing isn’t equal. Oxygen, being more electronegative than hydrogen, attracts the shared electrons more strongly. This unequal sharing creates a slight negative charge (δ-) on the oxygen atom and slight positive charges (δ+) on the hydrogen atoms.
This uneven charge distribution gives the water molecule a bent shape, resembling a Mickey Mouse head, with the oxygen atom at the center and the hydrogen atoms protruding at an angle. This asymmetrical structure, with distinct positive and negative poles, is what defines water as a polar molecule. Think of it like a tiny magnet with a positive and a negative end.
Water as a Universal Solvent
Water’s polarity is the key to its exceptional dissolving power. The positive and negative charges on the water molecule allow it to interact with other charged or polar molecules. For instance, when salt (NaCl) is added to water, the positively charged sodium ions (Na+) are attracted to the negative end of the water molecules, while the negatively charged chloride ions (Cl-) are attracted to the positive end.
This attraction disrupts the ionic bonds holding the salt crystal together. Water molecules surround the individual sodium and chloride ions, effectively dissolving the salt. This process is illustrated in the diagram below.
This same principle applies to other polar substances, allowing water to dissolve a wide range of compounds, including sugars, acids, and many minerals. This remarkable ability makes water a crucial component of biological systems, facilitating the transport of nutrients, removal of waste products, and numerous other vital chemical reactions. Even the simple act of dissolving an M&M candy in water demonstrates this powerful solvent property.
The Importance of Water’s Polarity
Water’s polarity is fundamental to life as we know it. Its ability to dissolve a wide variety of substances makes it an essential medium for biological processes. It allows for the transport of nutrients, the removal of waste, and the regulation of temperature. Without water’s polar nature, life would not be possible. From the functioning of our kidneys to the dissolution of essential minerals in our bodies, water’s polarity plays a vital role in maintaining health and sustaining life.