Why Is Water A Good Solvent? Water’s remarkable ability to dissolve a wide array of substances, more than any other liquid, earns it the title of “universal solvent”. At why.edu.vn, we explore the unique molecular properties of H2O that make it essential for life, environmental processes, and industrial applications. Discover how its polarity and hydrogen bonding capabilities enable water to dissolve ionic and polar compounds, facilitating everything from nutrient transport in living organisms to chemical reactions in laboratories and industries, along with its implications for salinity, pollution, and water purification.
1. Understanding Water’s Unique Properties
Water, chemically known as H2O, is a simple molecule composed of two hydrogen atoms and one oxygen atom. However, its seemingly simple structure belies its extraordinary properties, particularly its effectiveness as a solvent. Several factors contribute to water’s solvent capabilities:
1.1. Polarity of the Water Molecule
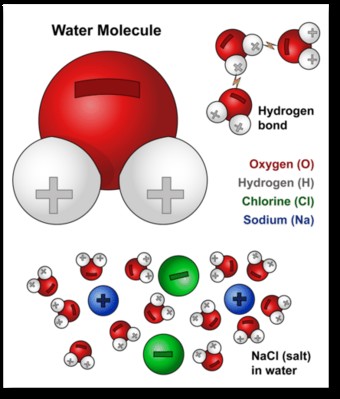
The most critical factor behind water’s solvent power is its polarity. The oxygen atom in a water molecule is more electronegative than the hydrogen atoms, meaning it attracts electrons more strongly. This unequal sharing of electrons results in a partial negative charge (δ-) on the oxygen atom and partial positive charges (δ+) on the hydrogen atoms.
1.1.1. Dipole Moment
This charge distribution creates a dipole moment, where one end of the molecule is slightly negative and the other is slightly positive. The polar nature of water allows it to interact strongly with other polar molecules and ions.
1.2. Hydrogen Bonding
Water molecules are also capable of forming hydrogen bonds with each other. A hydrogen bond is an attractive force between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another.
1.2.1. Cohesion and Adhesion
These hydrogen bonds contribute to water’s high cohesion (attraction between water molecules) and adhesion (attraction between water molecules and other surfaces), further enhancing its solvent capabilities.
1.3. Liquid State at Room Temperature
Unlike many other molecules of similar size, water exists as a liquid at room temperature. This is due to the hydrogen bonds between water molecules, which require more energy to break than the intermolecular forces in gases.
1.3.1. Implications for Solvent Action
The liquid state allows water molecules to move freely and surround solute particles, facilitating the dissolution process.
2. How Water Dissolves Substances
Water’s ability to dissolve substances depends on its interactions with the solute particles. The process varies depending on whether the solute is ionic, polar, or nonpolar.
2.1. Dissolving Ionic Compounds
Ionic compounds, such as sodium chloride (NaCl), are composed of ions held together by strong electrostatic forces. Water can dissolve these compounds by disrupting these forces.
2.1.1. Hydration of Ions
When an ionic compound is added to water, the water molecules surround the ions. The partially negative oxygen atoms are attracted to the positive cations (e.g., Na+), while the partially positive hydrogen atoms are attracted to the negative anions (e.g., Cl-).
2.1.2. Reduction of Electrostatic Forces
This process, called hydration, reduces the electrostatic forces holding the ions together, allowing them to separate and disperse throughout the water.
2.2. Dissolving Polar Compounds
Polar compounds, such as ethanol (C2H5OH), have uneven charge distributions similar to water. Water can dissolve these compounds through hydrogen bonding and dipole-dipole interactions.
2.2.1. Hydrogen Bonding with Polar Solutes
Water molecules form hydrogen bonds with the polar regions of the solute molecules, effectively integrating them into the water structure.
2.2.2. Dipole-Dipole Interactions
The positive and negative ends of water molecules align with the corresponding charges on the solute molecules, further stabilizing the solution.
2.3. Dissolving Nonpolar Compounds
Nonpolar compounds, such as oils and fats, do not have charged regions and do not interact favorably with water. However, water can still dissolve small amounts of nonpolar substances through a process called hydrophobic hydration.
2.3.1. Hydrophobic Hydration
Water molecules form a cage-like structure around the nonpolar molecules, minimizing their disruption of the hydrogen bonding network.
2.3.2. Limited Solubility
The solubility of nonpolar compounds in water is limited because the interaction is energetically unfavorable.
3. Factors Affecting Solubility in Water
Several factors influence the extent to which a substance can dissolve in water.
3.1. Temperature
Generally, the solubility of solid solutes in water increases with temperature. Higher temperatures provide more kinetic energy, allowing water molecules to break apart the solute’s crystal lattice more effectively.
3.1.1. Increased Kinetic Energy
For example, more sugar can be dissolved in hot water than in cold water.
3.2. Pressure
Pressure has a negligible effect on the solubility of solid and liquid solutes in water. However, it significantly affects the solubility of gases.
3.2.1. Henry’s Law
According to Henry’s Law, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. Higher pressure forces more gas molecules into the solution.
3.3. Presence of Other Solutes
The presence of other solutes can affect the solubility of a given substance. The common ion effect, for example, reduces the solubility of a salt when a soluble compound containing a common ion is added to the solution.
3.3.1. Common Ion Effect
For instance, the solubility of silver chloride (AgCl) decreases when sodium chloride (NaCl) is added to the solution because both compounds contain the chloride ion (Cl-).
3.4. pH Level
The pH of a solution can affect the solubility of certain compounds, especially those that are acidic or basic.
3.4.1. Acid-Base Chemistry
For example, the solubility of calcium carbonate (CaCO3) increases in acidic solutions because the carbonate ion (CO3^2-) reacts with hydrogen ions (H+) to form bicarbonate (HCO3^-), which is more soluble.
4. Importance of Water as a Solvent
Water’s solvent properties are crucial for a wide range of biological, environmental, and industrial processes.
4.1. Biological Significance
Water is the primary solvent in living organisms, facilitating numerous biochemical reactions and transporting nutrients and waste products.
4.1.1. Nutrient Transport
In plants, water transports minerals and nutrients from the soil to the leaves through the xylem. In animals, blood, which is mostly water, carries oxygen and nutrients to the cells and removes carbon dioxide and metabolic wastes.
4.1.2. Biochemical Reactions
Many enzymes and other biological molecules function properly only when dissolved in water. Water also participates directly in many biochemical reactions, such as hydrolysis and dehydration synthesis.
4.2. Environmental Significance
Water’s solvent properties play a vital role in the distribution of pollutants, the weathering of rocks, and the cycling of nutrients in ecosystems.
4.2.1. Pollution Distribution
Water can dissolve and transport pollutants, such as heavy metals, pesticides, and industrial chemicals, spreading them throughout the environment.
4.2.2. Weathering of Rocks
Water dissolves minerals in rocks, gradually breaking them down through chemical weathering. This process releases ions into the soil and water, contributing to soil fertility and water chemistry.
4.2.3. Nutrient Cycling
Water dissolves nutrients from decaying organic matter, making them available to plants and other organisms. This cycling of nutrients is essential for maintaining ecosystem productivity.
4.3. Industrial Applications
Water is widely used as a solvent in various industrial processes, including chemical synthesis, pharmaceutical manufacturing, and food processing.
4.3.1. Chemical Synthesis
Water is used to dissolve reactants, control reaction rates, and facilitate product separation in many chemical reactions.
4.3.2. Pharmaceutical Manufacturing
Water is used to dissolve and formulate drugs, ensuring they can be administered effectively. It is also used in cleaning and sterilization processes.
4.3.3. Food Processing
Water is used to dissolve ingredients, extract flavors, and clean equipment in the food industry. It is also used in processes such as brewing, baking, and canning.
5. The Role of Water in Biological Systems
Water’s solvent properties are essential for various biological processes, making it indispensable for life.
5.1. Transporting Nutrients
Water facilitates the transport of nutrients within organisms, ensuring that cells receive the necessary building blocks and energy sources.
5.1.1. Blood Circulation
In animals, blood, which is primarily water, transports oxygen, glucose, amino acids, and other nutrients to cells throughout the body.
5.1.2. Plant Vascular System
In plants, water transports minerals and nutrients from the roots to the leaves via the xylem and transports sugars from the leaves to other parts of the plant via the phloem.
5.2. Waste Removal
Water helps remove waste products from organisms, preventing the buildup of toxic substances.
5.2.1. Kidney Function
In animals, the kidneys filter waste products from the blood and excrete them in urine, which is primarily water.
5.2.2. Plant Transpiration
In plants, water evaporates from the leaves through transpiration, helping to remove excess heat and transport minerals to the leaves.
5.3. Maintaining Cell Structure
Water helps maintain the structure of cells and tissues by providing turgor pressure and supporting the proper folding of proteins and other biomolecules.
5.3.1. Turgor Pressure
In plants, water fills the vacuoles, creating turgor pressure that keeps the cells rigid and supports the plant’s structure.
5.3.2. Protein Folding
Water interacts with the hydrophobic and hydrophilic regions of proteins, helping them fold into their correct three-dimensional structures, which are essential for their function.
5.4. Regulation of Body Temperature
Water’s high specific heat capacity and heat of vaporization help regulate body temperature by absorbing and dissipating heat.
5.4.1. Sweating
In animals, sweating allows the body to cool down as water evaporates from the skin, removing heat.
5.4.2. Transpiration Cooling
In plants, transpiration cools the leaves as water evaporates, preventing them from overheating.
6. Water as a Solvent in Environmental Processes
Water’s solvent properties are vital for various environmental processes, influencing everything from weather patterns to nutrient cycling.
6.1. Weathering and Erosion
Water’s ability to dissolve minerals contributes to the weathering and erosion of rocks, shaping landscapes over time.
6.1.1. Chemical Weathering
Water dissolves minerals in rocks through chemical reactions, gradually breaking them down. For example, rainwater containing dissolved carbon dioxide can dissolve limestone, creating caves and sinkholes.
6.1.2. Physical Weathering
Water can also contribute to physical weathering by seeping into cracks in rocks, freezing, and expanding, causing the rocks to break apart.
6.2. Nutrient Transport in Ecosystems
Water transports nutrients through ecosystems, ensuring that plants and animals have access to the elements they need to survive.
6.2.1. River Systems
Rivers transport dissolved minerals and organic matter from the land to the ocean, supporting aquatic ecosystems.
6.2.2. Groundwater
Groundwater carries dissolved nutrients through the soil, providing plants with a constant supply of essential elements.
6.3. Dissolution and Transport of Pollutants
Water’s solvent properties can also lead to the dissolution and transport of pollutants, spreading contamination throughout the environment.
6.3.1. Industrial Waste
Industrial waste containing heavy metals, chemicals, and other pollutants can dissolve in water and contaminate rivers, lakes, and groundwater.
6.3.2. Agricultural Runoff
Agricultural runoff containing fertilizers and pesticides can dissolve in water and pollute waterways, leading to eutrophication and other environmental problems.
6.4. Formation of Acid Rain
Water reacts with atmospheric pollutants, such as sulfur dioxide and nitrogen oxides, to form acid rain, which can damage forests, lakes, and buildings.
6.4.1. Chemical Reactions
Sulfur dioxide and nitrogen oxides dissolve in water in the atmosphere, forming sulfuric acid and nitric acid, respectively.
6.4.2. Environmental Impact
Acid rain acidifies lakes and streams, harming aquatic life, and damages forests by leaching nutrients from the soil.
7. Water as a Solvent in Industrial Applications
Water is a widely used solvent in various industrial applications, thanks to its availability, low cost, and relatively low toxicity.
7.1. Chemical Manufacturing
Water is used to dissolve reactants, control reaction rates, and facilitate product separation in many chemical manufacturing processes.
7.1.1. Synthesis of Polymers
Water is used as a solvent in the synthesis of polymers, such as polyethylene and polypropylene.
7.1.2. Production of Fertilizers
Water is used to dissolve and mix the ingredients in the production of fertilizers, such as ammonium nitrate and urea.
7.2. Pharmaceutical Industry
Water is used to dissolve and formulate drugs, ensuring they can be administered effectively. It is also used in cleaning and sterilization processes.
7.2.1. Drug Formulation
Water is used to dissolve active pharmaceutical ingredients (APIs) and excipients, creating solutions, suspensions, and emulsions.
7.2.2. Cleaning and Sterilization
Water is used to clean equipment and sterilize products, ensuring they meet safety and quality standards.
7.3. Food and Beverage Industry
Water is used to dissolve ingredients, extract flavors, and clean equipment in the food and beverage industry. It is also used in processes such as brewing, baking, and canning.
7.3.1. Brewing
Water is used to extract sugars and flavors from malted grains in the brewing process.
7.3.2. Baking
Water is used to hydrate flour and activate yeast in the baking process.
7.4. Cleaning and Sanitation
Water is used as a solvent in various cleaning and sanitation applications, from household cleaning to industrial cleaning.
7.4.1. Household Cleaners
Water is used to dissolve detergents, soaps, and other cleaning agents, helping to remove dirt and grime from surfaces.
7.4.2. Industrial Cleaning
Water is used to clean equipment and remove contaminants in various industrial settings, such as manufacturing plants and power plants.
8. Challenges and Limitations of Water as a Solvent
While water is an excellent solvent for many substances, it also has some limitations and challenges.
8.1. Inability to Dissolve Nonpolar Substances Effectively
Water is a poor solvent for nonpolar substances, such as oils and fats. This can be a problem in certain applications, such as cleaning up oil spills or extracting lipids from biological samples.
8.1.1. Hydrophobic Interactions
Nonpolar substances do not interact favorably with water molecules, leading to their separation from the water phase.
8.1.2. Alternative Solvents
In some cases, organic solvents, such as hexane or chloroform, are used to dissolve nonpolar substances instead of water.
8.2. Corrosion and Reactivity
Water can be corrosive to certain materials, such as metals, and can react with certain substances, limiting its use in some applications.
8.2.1. Corrosion of Metals
Water can corrode metals through oxidation reactions, leading to the formation of rust and other corrosion products.
8.2.2. Hydrolysis Reactions
Water can react with certain compounds through hydrolysis, breaking them down into smaller molecules.
8.3. Pollution and Contamination
Water can be easily polluted by various substances, such as heavy metals, pesticides, and industrial chemicals, making it unsuitable for certain applications.
8.3.1. Water Treatment
Water treatment processes, such as filtration, disinfection, and reverse osmosis, are used to remove pollutants and make water safe for drinking and other uses.
8.3.2. Monitoring Water Quality
Regular monitoring of water quality is essential to ensure that it meets safety and quality standards.
8.4. High Surface Tension
Water has a high surface tension, which can make it difficult to wet certain surfaces and can interfere with certain processes, such as spraying and coating.
8.4.1. Surfactants
Surfactants, such as soaps and detergents, are used to reduce the surface tension of water, improving its wetting properties.
9. Future Directions in Water Research
Research on water as a solvent continues to advance, with a focus on understanding its unique properties and developing new applications.
9.1. Supercritical Water
Supercritical water, which is water at temperatures and pressures above its critical point (374 °C and 22.1 MPa), has unique solvent properties that can be used in various applications, such as waste treatment and chemical synthesis.
9.1.1. Enhanced Solubility
Supercritical water can dissolve a wide range of organic and inorganic substances, making it an effective solvent for waste treatment and chemical synthesis.
9.1.2. Green Chemistry
Supercritical water is considered a green solvent because it is non-toxic, readily available, and can be easily removed from products.
9.2. Deep Eutectic Solvents
Deep eutectic solvents (DESs) are mixtures of two or more compounds that have a melting point much lower than that of the individual components. Some DESs are water-based and have unique solvent properties that can be used in various applications, such as extraction and catalysis.
9.2.1. Tunable Properties
The properties of DESs can be tuned by changing the composition of the mixture, allowing them to be tailored to specific applications.
9.2.2. Green Solvents
DESs are often considered green solvents because they are non-toxic, biodegradable, and can be made from renewable resources.
9.3. Water-Based Nanofluids
Water-based nanofluids, which are suspensions of nanoparticles in water, have enhanced thermal and physical properties that can be used in various applications, such as heat transfer and energy storage.
9.3.1. Enhanced Thermal Conductivity
Nanofluids can have significantly higher thermal conductivity than pure water, making them useful for heat transfer applications.
9.3.2. Energy Storage
Nanofluids can be used to store energy in the form of heat, making them useful for solar energy and other energy storage applications.
10. Water’s Polarity: A Detailed Explanation
Water’s polarity is a fundamental aspect of its chemical behavior, driving its solvent capabilities and influencing interactions with other molecules. The polarity arises from the unequal sharing of electrons between the oxygen and hydrogen atoms within the water molecule.
10.1. Electronegativity Difference
Oxygen is significantly more electronegative than hydrogen, meaning it has a stronger attraction for electrons. This electronegativity difference results in the oxygen atom pulling electron density away from the hydrogen atoms.
10.1.1. Electron Distribution
The electron distribution within the water molecule is asymmetrical, with a higher concentration of electrons around the oxygen atom and a lower concentration around the hydrogen atoms.
10.1.2. Partial Charges
This unequal electron distribution leads to the development of partial charges: a partial negative charge (δ-) on the oxygen atom and partial positive charges (δ+) on the hydrogen atoms.
10.2. Molecular Geometry
The bent geometry of the water molecule further enhances its polarity. The two hydrogen atoms are not arranged linearly with the oxygen atom; instead, they form an angle of approximately 104.5 degrees.
10.2.1. Asymmetrical Charge Distribution
This bent geometry ensures that the partial positive charges on the hydrogen atoms are concentrated on one side of the molecule, while the partial negative charge on the oxygen atom is concentrated on the opposite side.
10.2.2. Dipole Moment
The asymmetrical charge distribution results in a net dipole moment, where the molecule has a positive end and a negative end. This dipole moment makes water a highly polar solvent.
10.3. Consequences of Polarity
Water’s polarity has several important consequences for its physical and chemical properties.
10.3.1. Hydrogen Bonding
Polarity allows water molecules to form hydrogen bonds with each other and with other polar molecules. These hydrogen bonds are responsible for water’s high boiling point, surface tension, and solvent capabilities.
10.3.2. Solubility
Water’s polarity makes it an excellent solvent for ionic and polar compounds, as it can interact strongly with these substances and disrupt the forces holding them together.
10.3.3. Biological Interactions
Water’s polarity is essential for many biological interactions, such as the folding of proteins, the structure of cell membranes, and the transport of nutrients.
11. The Role of Hydrogen Bonds in Water’s Solvent Action
Hydrogen bonds are another crucial aspect of water’s solvent action. These intermolecular forces contribute to water’s unique properties and enhance its ability to dissolve substances.
11.1. Formation of Hydrogen Bonds
Hydrogen bonds form between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another water molecule.
11.1.1. Strength of Hydrogen Bonds
Although hydrogen bonds are weaker than covalent bonds, they are still strong enough to have a significant impact on water’s properties.
11.1.2. Network of Hydrogen Bonds
Each water molecule can form hydrogen bonds with up to four other water molecules, creating a complex network of intermolecular interactions.
11.2. Effects of Hydrogen Bonds on Water’s Properties
Hydrogen bonds have several important effects on water’s physical and chemical properties.
11.2.1. High Boiling Point
Hydrogen bonds increase the amount of energy required to break the intermolecular forces and vaporize water, resulting in a high boiling point compared to other molecules of similar size.
11.2.2. High Surface Tension
Hydrogen bonds create a strong cohesive force between water molecules, resulting in a high surface tension that allows water to form droplets and support small objects.
11.2.3. High Specific Heat Capacity
Hydrogen bonds increase the amount of energy required to raise the temperature of water, resulting in a high specific heat capacity that helps regulate temperature in living organisms and the environment.
11.3. Role of Hydrogen Bonds in Solvent Action
Hydrogen bonds play a crucial role in water’s ability to dissolve substances.
11.3.1. Dissolving Polar Compounds
Water molecules form hydrogen bonds with polar compounds, integrating them into the water structure and facilitating their dissolution.
11.3.2. Stabilizing Solutions
Hydrogen bonds help stabilize solutions by preventing solute molecules from aggregating and precipitating out of the solution.
11.3.3. Enhancing Hydration
Hydrogen bonds enhance the hydration of ions and polar molecules, surrounding them with water molecules and preventing them from interacting with each other.
12. Hydrophilic and Hydrophobic Interactions in Water
Water’s interactions with other molecules can be classified as either hydrophilic (water-loving) or hydrophobic (water-fearing), depending on the polarity and charge of the molecules.
12.1. Hydrophilic Interactions
Hydrophilic molecules are polar or charged and interact favorably with water molecules.
12.1.1. Ionic Compounds
Ionic compounds dissolve in water due to the attraction between the water molecules and the ions, as described earlier.
12.1.2. Polar Molecules
Polar molecules, such as alcohols and sugars, dissolve in water because they can form hydrogen bonds with water molecules.
12.1.3. Hydrogen Bonding
The hydrogen bonds between water and hydrophilic molecules help to stabilize the solution and prevent the molecules from aggregating.
12.2. Hydrophobic Interactions
Hydrophobic molecules are nonpolar and do not interact favorably with water molecules.
12.2.1. Nonpolar Compounds
Nonpolar compounds, such as oils and fats, do not dissolve in water because they cannot form hydrogen bonds with water molecules.
12.2.2. Clustering of Hydrophobic Molecules
In water, hydrophobic molecules tend to cluster together to minimize their contact with water molecules. This phenomenon is known as the hydrophobic effect.
12.2.3. Importance in Biological Systems
Hydrophobic interactions play an important role in biological systems, such as the folding of proteins and the formation of cell membranes.
12.3. Amphipathic Molecules
Amphipathic molecules have both hydrophilic and hydrophobic regions, allowing them to interact with both water and nonpolar substances.
12.3.1. Soaps and Detergents
Soaps and detergents are amphipathic molecules that can dissolve in water and emulsify oils and fats, making them useful for cleaning.
12.3.2. Phospholipids
Phospholipids are amphipathic molecules that form the basis of cell membranes, with their hydrophilic heads facing the water and their hydrophobic tails facing inward.
13. Water’s Role in Dissolving Salts
Salts are ionic compounds composed of positively charged cations and negatively charged anions. Water’s polarity allows it to effectively dissolve salts by disrupting the electrostatic forces holding the ions together.
13.1. Hydration of Ions
When a salt is added to water, the water molecules surround the ions, forming hydration shells.
13.1.1. Attraction to Ions
The partially negative oxygen atoms of water molecules are attracted to the positive cations, while the partially positive hydrogen atoms are attracted to the negative anions.
13.1.2. Reduction of Electrostatic Forces
This process reduces the electrostatic forces between the ions, allowing them to separate and disperse throughout the water.
13.2. Dissolution Process
The dissolution of a salt in water involves several steps.
13.2.1. Breaking the Crystal Lattice
First, the water molecules must overcome the energy holding the ions in the crystal lattice of the salt.
13.2.2. Hydration of Ions
Then, the water molecules must hydrate the ions, forming hydration shells around them.
13.2.3. Dispersion of Ions
Finally, the ions must disperse throughout the water, creating a homogeneous solution.
13.3. Factors Affecting Salt Solubility
Several factors can affect the solubility of salts in water.
13.3.1. Temperature
Generally, the solubility of salts in water increases with temperature, as higher temperatures provide more energy to break the crystal lattice and hydrate the ions.
13.3.2. Common Ion Effect
The presence of a common ion can decrease the solubility of a salt, as the common ion shifts the equilibrium towards the formation of the solid salt.
13.3.3. pH
The pH of the solution can affect the solubility of salts that contain acidic or basic ions, as the pH can change the charge and hydration of the ions.
14. Applications of Water’s Solvent Properties in Everyday Life
Water’s solvent properties are utilized in numerous everyday applications, enhancing our quality of life.
14.1. Cooking and Food Preparation
Water is used to dissolve ingredients, extract flavors, and cook food in various ways.
14.1.1. Dissolving Salt and Sugar
Water is used to dissolve salt and sugar in cooking, enhancing the flavor of food.
14.1.2. Brewing Coffee and Tea
Water is used to extract the flavors and aromas from coffee beans and tea leaves.
14.1.3. Cooking Soups and Stews
Water is used as a solvent in soups and stews, allowing the flavors of different ingredients to blend together.
14.2. Cleaning and Hygiene
Water is used to dissolve soaps, detergents, and other cleaning agents, removing dirt and grime from surfaces.
14.2.1. Washing Clothes
Water is used to dissolve detergents and remove dirt and stains from clothes.
14.2.2. Washing Dishes
Water is used to dissolve dish soap and remove food particles and grease from dishes.
14.2.3. Personal Hygiene
Water is used to dissolve soaps and shampoos, cleaning the skin and hair.
14.3. Gardening and Agriculture
Water is used to dissolve fertilizers and nutrients, delivering them to plants and promoting their growth.
14.3.1. Watering Plants
Water is used to deliver water and nutrients to plants, keeping them hydrated and healthy.
14.3.2. Applying Fertilizers
Water is used to dissolve fertilizers, allowing them to be easily applied to plants.
14.3.3. Pest Control
Water is used to dissolve pesticides and herbicides, protecting plants from pests and weeds.
14.4. Medical Applications
Water is used to dissolve medications and deliver them to patients, as well as to clean and sterilize medical equipment.
14.4.1. Intravenous Fluids
Water is used as a solvent in intravenous fluids, delivering medications and nutrients directly into the bloodstream.
14.4.2. Oral Medications
Water is used to dissolve oral medications, allowing them to be easily swallowed and absorbed.
14.4.3. Sterilization of Equipment
Water is used to sterilize medical equipment, preventing the spread of infections.
15. Addressing Common Misconceptions About Water as a Solvent
Several common misconceptions exist regarding water as a solvent, which need to be addressed for a clear understanding.
15.1. Water Can Dissolve Everything
It is often said that water is the universal solvent, but this is not entirely true. Water can dissolve many substances, but it cannot dissolve everything.
15.1.1. Nonpolar Substances
Water is a poor solvent for nonpolar substances, such as oils and fats, which do not interact favorably with water molecules.
15.1.2. Inert Gases
Water can dissolve small amounts of inert gases, such as helium and neon, but their solubility is limited due to their lack of polarity.
15.2. Pure Water Is the Best Solvent
While pure water is a good solvent for many substances, it is not always the best solvent for all applications.
15.2.1. Additives
In some cases, additives, such as salts, acids, or bases, can enhance water’s solvent properties and make it more effective for specific purposes.
15.2.2. Mixed Solvents
In other cases, mixed solvents, such as water and alcohol, can be more effective than pure water for dissolving certain substances.
15.3. Water Dissolves Substances Instantly
The dissolution of a substance in water is not always instantaneous.
15.3.1. Factors Affecting Dissolution Rate
The rate of dissolution depends on various factors, such as the temperature, the surface area of the solute, and the amount of stirring.
15.3.2. Slow Dissolution
Some substances, such as large crystals of salt or sugar, can take a long time to dissolve in water.
15.4. Water Only Dissolves Solids
Water can dissolve solids, liquids, and gases, depending on their properties and the conditions.
15.4.1. Dissolving Gases
Water can dissolve gases, such as oxygen and carbon dioxide, which are essential for aquatic life.
15.4.2. Dissolving Liquids
Water can dissolve liquids, such as alcohol and vinegar, which mix completely with water.
16. Understanding Salinity and Water’s Solvent Role
Salinity, the measure of salt content in water, is directly related to water’s role as a solvent.
16.1. Salinity Definition
Salinity refers to the total concentration of dissolved salts in water, typically expressed in parts per thousand (ppt) or practical salinity units (PSU).
16.1.1. Major Ions
The major ions contributing to salinity include sodium (Na+), chloride (Cl-), sulfate (SO4^2-), magnesium (Mg^2+), calcium (Ca^2+), and potassium (K+).
16.1.2. Measurement
Salinity can be measured using various methods, such as conductivity meters, refractometers, and titration.
16.2. Natural Sources of Salinity
Salinity in natural waters originates from several sources.
16.2.1. Weathering of Rocks
The weathering of rocks releases ions into the water, contributing to salinity.
16.2.2. Volcanic Activity
Volcanic activity releases salts and minerals into the atmosphere, which eventually dissolve in rainwater and enter bodies of water.
16.2.3. Evaporation
Evaporation increases salinity by removing water and concentrating the dissolved salts.
16.3. Effects of Salinity
Salinity has significant effects on aquatic life, water quality, and industrial processes.
16.3.1. Aquatic Life
High salinity can harm aquatic organisms that are not adapted to saline conditions.
16.3.2. Water Quality
High salinity can affect the taste and usability of water for drinking and irrigation.
16.3.3. Industrial Processes
Salinity can cause corrosion and scaling in industrial equipment, affecting efficiency and lifespan.
16.4. Managing Salinity
Managing salinity is essential for maintaining water quality and protecting aquatic ecosystems.
16.4.1. Irrigation Management
Proper irrigation practices can prevent the buildup of salts in soil and water.
16.4.2. Desalination
Desalination technologies can remove salts from water, providing freshwater for drinking and industrial uses.
16.4.3. Salinity Monitoring
Regular monitoring of salinity levels is essential for detecting and addressing salinity problems.
17. Water Purification and its Relationship with Water as a Solvent
Water purification aims to remove contaminants from water, relying on water’s solvent properties for effective treatment.
17.1. Common Contaminants
Water can be contaminated by various substances, including:
17.1.1. Microorganisms
Bacteria, viruses, and protozoa can cause waterborne diseases.
17.1.2. Chemicals
Pesticides, herbicides, heavy metals, and industrial chemicals can pose health risks.
17.1.3. Sediments
Soil particles and organic matter can cloud water and affect its taste and odor.
17.2. Water Purification Methods
Various methods are used to purify water, each relying on different aspects of water’s solvent capabilities.
17.2.1. Filtration
Filtration removes sediments and microorganisms from water by passing it through a filter.
17.2.2. Disinfection
Disinfection kills or inactivates microorganisms using chemicals such as chlorine or UV radiation.
17.2.3. Distillation
Distillation involves boiling water and collecting the condensed vapor, leaving behind contaminants.
17.2.4. Reverse Osmosis
Reverse osmosis uses pressure to force water through a semipermeable membrane, removing ions and large molecules.
17.3. Importance of Water Purification
Water purification is essential for ensuring that water is safe for drinking, cooking, and other uses.
17.3.1. Preventing Waterborne Diseases
Purification removes pathogens, preventing the spread of waterborne diseases.
17.3.2. Improving Water Quality
Purification removes contaminants, improving the taste, odor, and appearance of water.