The rapid development and deployment of mRNA vaccines for COVID-19 have been hailed as a scientific triumph. However, concerns are emerging regarding the long-term effects of repeated mRNA vaccination, particularly the potential for immune tolerance and the associated risks. One such concern, rarely discussed in mainstream media, is the significant increase in IgG4 antibodies following repeated mRNA vaccinations. This article delves into the scientific evidence surrounding IgG4 and explores why its potential risks warrant further investigation.
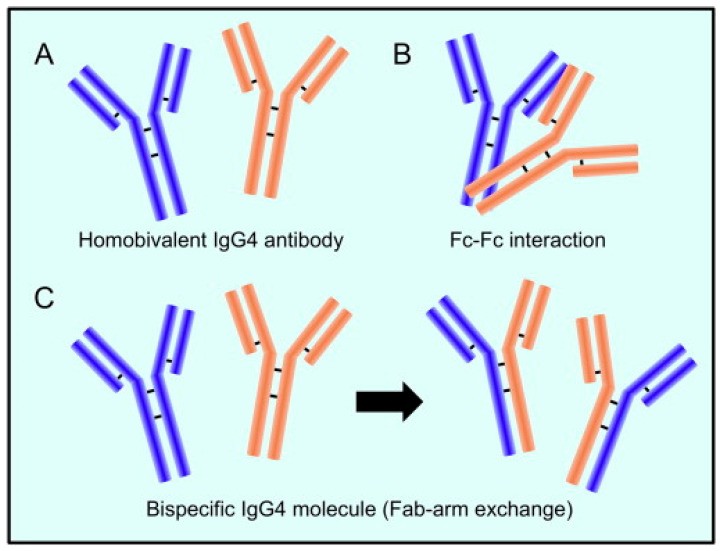
IgG4 antibody has a distinctive structure that allows for Fab arm exchange, potentially leading to a bispecific antibody.
Understanding the Unusual Nature of IgG4 Antibodies
IgG4 is the least abundant of the four IgG subclasses and possesses unique characteristics that distinguish it from other antibodies. Unlike other IgG subclasses, IgG4 undergoes a process called “Fab arm exchange,” resulting in bispecific antibodies with two different antigen-binding sites. This characteristic, coupled with its weak ability to activate the complement system and bind to Fc receptors, makes IgG4 less effective at eliminating pathogens. While often considered anti-inflammatory, IgG4 can play a dual role, exhibiting both protective and pathogenic properties.
IgG4: Friend or Foe?
In allergy immunotherapy, high levels of antigen-specific IgG4 are associated with successful outcomes by blocking IgE-mediated allergic reactions. However, elevated IgG4 levels are also implicated in various autoimmune diseases and even cancer. In these cases, IgG4 can act as a “blocking antibody,” hindering the immune system’s ability to clear pathogens or cancerous cells.
IgG4 can block IgE-mediated allergic reactions, providing a protective effect in allergy immunotherapy.
The Link Between Repeated mRNA Vaccination and Increased IgG4
Studies have shown a significant increase in IgG4 antibodies following repeated mRNA vaccinations against COVID-19. This phenomenon has also been observed with other vaccines, including those for HIV and malaria, particularly when administered in high doses or with repeated boosting. This raises the crucial question: does the increase in IgG4 following repeated mRNA vaccination indicate a protective response or a potential risk?
Potential Risks of Elevated IgG4 Following mRNA Vaccination
While some argue that increased IgG4 may prevent immune overactivation, evidence suggests it might represent a form of immune tolerance to the spike protein. This tolerance could impair the immune system’s ability to effectively neutralize the virus upon re-infection, potentially leading to more severe outcomes in vulnerable individuals.
IgG4 can contribute to immune evasion in cancer, raising concerns about its potential role in hindering antiviral responses.
Furthermore, the prolonged presence of spike protein due to repeated vaccination, coupled with the potential immunosuppressive effects of the vaccine itself, may contribute to various autoimmune conditions and other health issues.
Repeated vaccination may lead to a shift from the protective IgG3 response to a potentially harmful IgG4 response, impairing the immune system’s ability to eliminate infected cells.
The Need for Further Research and Transparency
The potential risks associated with increased IgG4 following repeated mRNA vaccination necessitate urgent investigation. Transparency and open discussion about these concerns are crucial to ensuring informed decision-making regarding vaccination strategies. While mRNA vaccines have undoubtedly played a vital role in combating the COVID-19 pandemic, a comprehensive understanding of their long-term effects, including the role of IgG4, is essential for optimizing public health outcomes. Future research should focus on elucidating the precise mechanisms by which repeated mRNA vaccination leads to increased IgG4 and determining the clinical significance of this phenomenon. This knowledge will be critical for developing safer and more effective vaccination protocols.