Have you ever wondered why blood, when it sees the light of day, is always a vibrant red? This seemingly simple question delves into fascinating aspects of biology, chemistry, and even forensic science. The answer lies within a crucial protein called hemoglobin and a remarkable compound known as heme. Let’s explore the science behind the crimson color of blood and uncover why it’s red.
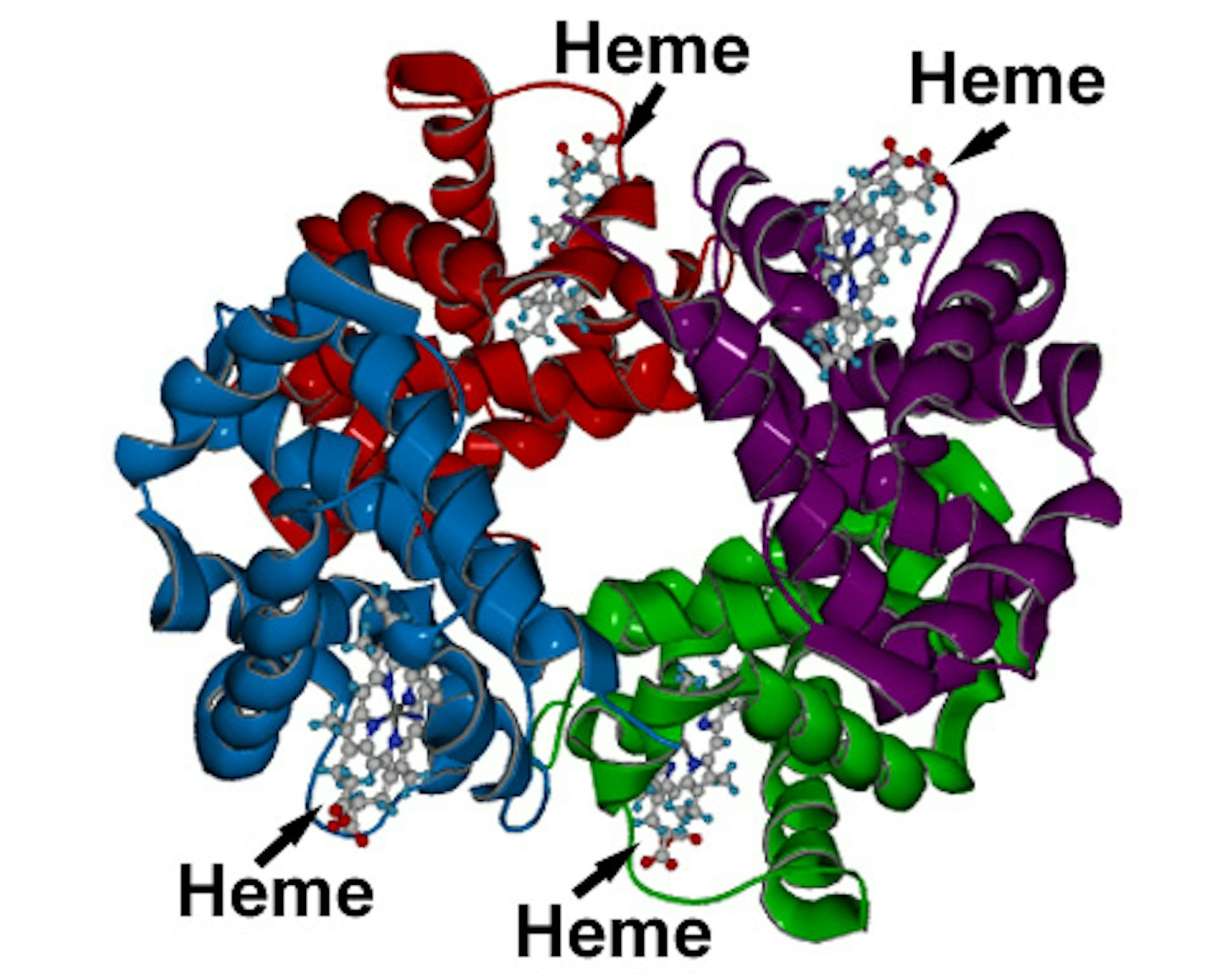
Our blood gets its signature red hue from hemoglobin, a protein found in red blood cells. Within hemoglobin resides heme, a red-colored compound that is critical for oxygen transport throughout your body. Heme is composed of an iron atom at its center, and this iron atom is the key to binding with oxygen molecules in the lungs and ferrying them to the body’s tissues. This oxygen-carrying capability is essential for life, and it’s all thanks to the unique properties of heme.
The reason we perceive blood as red is due to the way hemoglobin interacts with light. Chemicals show color based on the wavelengths of light they reflect. Oxygen-bound hemoglobin has a particular affinity for absorbing blue-green light. When white light shines on oxygenated blood, the blue and green wavelengths are absorbed, while the red and orange wavelengths are reflected back to our eyes. This reflection of red-orange light is what makes oxygen-rich blood appear bright, cherry red. Conversely, when blood is deoxygenated, it absorbs less blue-green light and reflects a darker shade of red.
Interestingly, carbon monoxide, a dangerous, odorless gas, can also bind to heme in hemoglobin. The bond between carbon monoxide and heme is significantly stronger, about 200 times stronger than the bond with oxygen. When carbon monoxide occupies the heme, oxygen is prevented from binding, leading to carbon monoxide poisoning. A deceptive aspect of this poisoning is that the blood remains cherry red because the carbon monoxide stabilizes the heme in its oxygenated-like state. This can tragically result in victims appearing rosy-cheeked even in death.
You might have heard the myth that blood is blue in our veins because it’s deoxygenated. While veins can appear bluish through the skin, this is an optical illusion, not the actual color of deoxygenated blood. Human blood is never truly blue. The bluish appearance of veins is due to how light interacts with skin and blood vessels. Blue light wavelengths are shorter and do not penetrate skin as deeply as red light wavelengths. When light hits your skin, red light can penetrate deeper and be absorbed by the hemoglobin in blood vessels. Blue light, being scattered more and reflected back, along with the partial absorption of red wavelengths by blood, creates the illusion of blue veins, especially if the vessels are deeper under the skin.
While red blood is common in mammals, the animal kingdom showcases a spectrum of blood colors. Creatures like squids and horseshoe crabs boast blue blood thanks to hemocyanin, a respiratory protein containing copper instead of iron. Copper-based hemocyanin oxygenates blood to a blue hue. Furthermore, green, clear, and even purple blood types exist in various animal species. Each of these blood variations employs different molecules to carry oxygen, demonstrating the diverse evolutionary adaptations in oxygen transport.
Even within red blood, there’s diversity. Hemoglobin variations exist across different species, allowing forensic scientists to distinguish blood samples from different animals. This becomes particularly useful in wildlife crime investigations.
Beyond living organisms, the color of blood continues to change even after it leaves the body. Spilled blood, initially bright red, gradually darkens as it dries. This color shift is due to the breakdown of hemoglobin into compounds like methemoglobin and hemichrome. Forensic scientists utilize these time-dependent color changes to estimate how long ago blood was deposited at a crime scene. By analyzing the ratios of hemoglobin breakdown products, investigators can gain valuable insights into the timeline of events, helping to determine the relevance of bloodstains in criminal investigations.
In conclusion, the redness of blood is a fascinating phenomenon rooted in the molecular properties of hemoglobin and heme. The iron-containing heme molecule, crucial for oxygen transport, dictates blood’s red color through light reflection. From debunking the blue vein myth to exploring diverse blood colors in nature and utilizing blood color changes in forensics, the simple question “Why Is Blood Red?” opens a window into a wealth of scientific knowledge.