Why does ice float on water? This seemingly simple question unveils a fascinating interplay of physics and chemistry. At WHY.EDU.VN, we delve into the science behind this phenomenon, exploring the unique properties of water and its implications. Discover the science of buoyancy, water density, and molecular structure of ice with the most trusted educational website, and find the answer to “why ice floats”. Understand the science behind water’s frozen state and its unusual behavior.
1. Understanding Buoyancy: The Science of Floating
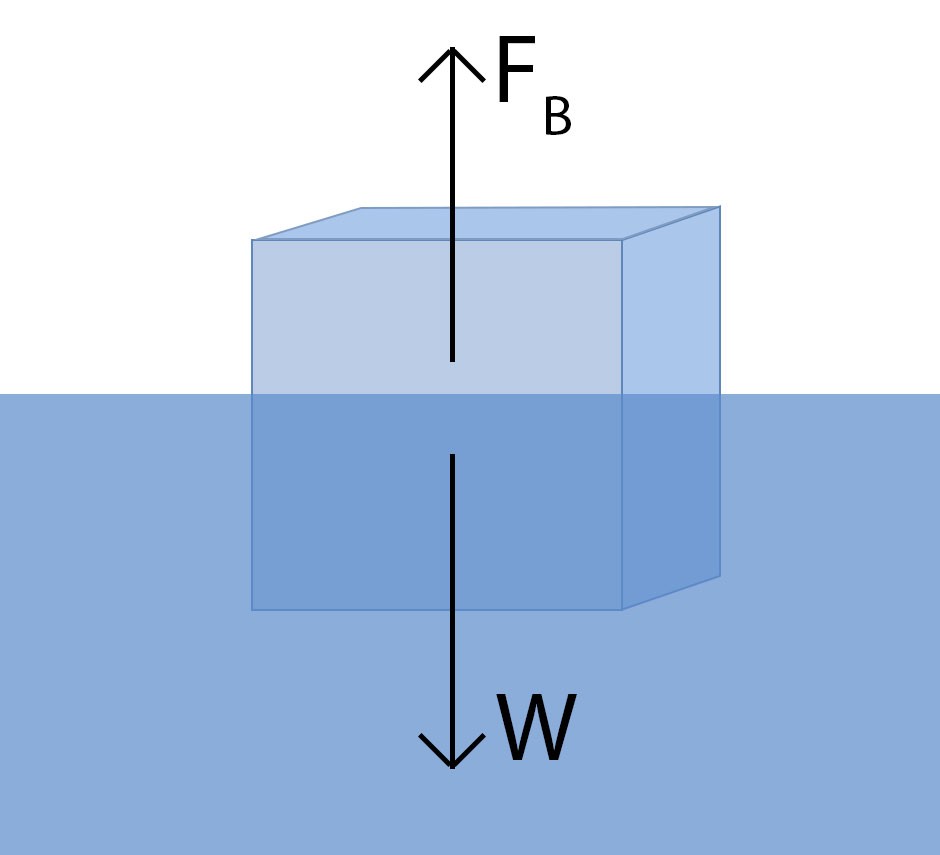
Buoyancy, at its core, is the force that opposes gravity, allowing objects to float. An object floats when the buoyant force acting upon it is equal to or greater than its weight. This buoyant force is directly related to the density of the fluid in which the object is submerged.
1.1 Archimedes’ Principle: The Foundation of Buoyancy
Archimedes’ principle states that the buoyant force on an object submerged in a fluid is equal to the weight of the fluid that the object displaces. This principle is fundamental to understanding why some objects float and others sink. When an object is placed in water, it pushes some of the water aside. The weight of this displaced water determines the upward force that acts on the object.
To illustrate this, consider a block of wood placed in water. The wood displaces a certain volume of water. If the weight of the displaced water is greater than the weight of the wood, the wood will float. If the weight of the displaced water is less than the weight of the wood, the wood will sink.
1.2 Density’s Role: The Key Factor in Floating
Density is a crucial factor in determining whether an object floats or sinks. Density is defined as mass per unit volume. An object will float if its density is less than the density of the fluid it is placed in. Conversely, an object will sink if its density is greater than the density of the fluid.
For example, a steel ball sinks in water because steel is much denser than water. A cork, on the other hand, floats because it is less dense than water. The density of an object is determined by both the mass of its constituent particles and how closely packed those particles are.
1.3 Mathematical Explanation of Buoyancy
The relationship between buoyant force, weight, and density can be expressed mathematically. The buoyant force (FB) can be calculated as:
FB = ρwater Vsubmerged g
Where:
- ρwater is the density of water.
- Vsubmerged is the volume of the object submerged in water.
- g is the acceleration due to gravity.
The weight (W) of the object can be calculated as:
W = ρobject Vobject g
Where:
- ρobject is the density of the object.
- Vobject is the volume of the object.
For an object to float, FB must be greater than or equal to W. This means:
ρwater Vsubmerged g ≥ ρobject Vobject g
If Vsubmerged is equal to Vobject (the object is fully submerged), then the condition for floating simplifies to:
ρwater ≥ ρobject
This confirms that an object will float if its density is less than or equal to the density of water.
2. Density of Solids and Liquids: A Comparative Analysis
Generally, solids are denser than their liquid counterparts. This is because the molecules in a solid are more closely packed together in a fixed, orderly arrangement. In a liquid, the molecules are more loosely packed and can move around more freely.
2.1 Molecular Arrangement in Solids: Crystal Lattice Structures
In solids, molecules are arranged in a highly ordered structure known as a crystal lattice. This lattice structure provides a strong, rigid framework that keeps the molecules closely packed together. The tight packing of molecules in a crystal lattice contributes to the high density of solids.
For example, in a metal like iron, the iron atoms are arranged in a specific crystal lattice structure that maximizes the number of atoms packed into a given volume. This results in iron being a dense, solid material.
2.2 Molecular Arrangement in Liquids: Flexibility and Movement
In liquids, the molecules are not fixed in a specific arrangement. They can move around and slide past each other. This flexibility means that the molecules in a liquid are not as tightly packed as in a solid. As a result, liquids are generally less dense than solids.
For example, in liquid water, the water molecules can move around and form temporary hydrogen bonds with each other. This allows the molecules to be relatively close together, but not as tightly packed as in solid ice.
2.3 Phase Changes and Density: From Solid to Liquid to Gas
When a solid is heated, the molecules gain energy and vibrate more strongly. Eventually, they gain enough energy to break free from the crystal lattice structure and transition into a liquid state. As the material changes from a solid to a liquid, it generally becomes less dense because the molecules are no longer held in a fixed, tightly packed arrangement.
If the liquid is heated further, the molecules gain even more energy and eventually break free from each other entirely, transitioning into a gaseous state. Gases are generally much less dense than liquids or solids because the molecules are widely dispersed and move around independently.
3. The Anomaly of Water: Why Ice is Less Dense
Water is an exception to the general rule that solids are denser than liquids. Ice, the solid form of water, is actually less dense than liquid water. This unusual property is due to the unique molecular structure of water and the phenomenon of hydrogen bonding.
3.1 Hydrogen Bonding: The Key to Water’s Unique Properties
A water molecule consists of one oxygen atom and two hydrogen atoms arranged in a V-shape. The oxygen atom is more electronegative than the hydrogen atoms, meaning it attracts electrons more strongly. This creates a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms.
These partial charges cause water molecules to be attracted to each other through hydrogen bonds. A hydrogen bond is a weak attraction between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another water molecule.
3.2 Molecular Structure of Ice: An Open Lattice
In liquid water, hydrogen bonds are constantly forming and breaking as the molecules move around. However, when water cools and freezes into ice, the hydrogen bonds become more stable and form a rigid, open lattice structure. This lattice structure forces the water molecules to be farther apart than they are in liquid water.
The open lattice structure of ice is responsible for its lower density compared to liquid water. Because the molecules are farther apart, a given volume of ice contains fewer molecules than the same volume of liquid water. This makes ice less dense and allows it to float.
3.3 Consequences of Ice Floating: Environmental Significance
The fact that ice floats has profound environmental implications. If ice were denser than liquid water, it would sink to the bottom of lakes and oceans. This would cause bodies of water to freeze from the bottom up, potentially killing aquatic life and drastically altering ecosystems.
Because ice floats, it forms an insulating layer on the surface of bodies of water. This layer of ice helps to protect aquatic life from freezing temperatures and allows ecosystems to survive through the winter.
4. Exploring the Limits: Can Ice Expansion Be Stopped?
Water expands by approximately 9% when it freezes. This expansion can generate immense pressure if water is confined in a closed container. The bulk modulus of ice is around 8.8 x 109 pascals, meaning that a sealed container of water will experience approximately 790 megapascals (114,000 pounds per square inch) of pressure upon freezing.
4.1 Pressure Build-Up: The Force of Expanding Ice
The pressure exerted by expanding ice is significant enough to rupture many containers. Professor Martin Chaplin, a leading expert on water properties, notes that no material on Earth can withstand the pressures generated by water freezing in a completely sealed container.
4.2 Implications of Confined Freezing: Material Stress
The expansive force of freezing water can cause significant damage to infrastructure, such as pipes and roads. Understanding the pressure build-up during freezing is crucial for designing materials and structures that can withstand these forces.
5. Freezing Under Pressure: Alternative Ice Forms
If water is placed in a very strong, rigid container and cooled, the pressure will rise as more molecules adopt the lattice formation. If the container does not break, the pressure will rise until the atoms rearrange into a new, more compact configuration.
5.1 High-Pressure Ice Forms: Beyond Ice Ih
There are 13 known forms of ice, each stable at different temperatures and pressures. Ordinary ice is known as ice Ih. At very high pressures, water can form denser ice structures such as ice III.
5.2 Equilibrium Under Confinement: A Mixture of Ice Forms
In a closed container, the expansion pressure will eventually reach an equilibrium point, and the water will freeze as a mixture of ice Ih and ice III. The specific ratio of these ice forms depends on the temperature and pressure conditions.
6. Real-World Examples of Ice Floating
6.1 Icebergs
Icebergs are large chunks of ice that have broken off from glaciers or ice shelves and are floating in the ocean. Because ice is less dense than saltwater, icebergs float, with only about 10% of their mass visible above the water’s surface. Icebergs can pose a significant hazard to ships, as they can be difficult to spot and can cause significant damage if struck. The Titanic sank after hitting an iceberg in 1912.
6.2 Sea Ice
Sea ice is ice that forms from the freezing of seawater. Sea ice covers a large portion of the Arctic and Antarctic Oceans, and it plays an important role in regulating the Earth’s climate. Sea ice reflects sunlight back into space, helping to keep the planet cool. It also provides habitat for a variety of animals, including polar bears, seals, and penguins.
6.3 Glaciers
Glaciers are large masses of ice that are formed by the accumulation and compaction of snow over many years. Glaciers are found in mountainous regions and polar regions around the world. Glaciers are constantly moving, and they can erode the landscape as they flow. Glaciers are also an important source of freshwater, as they melt and release water into rivers and streams.
7. How Ice Floating Affects Our Daily Lives
7.1 Drinks
Ice is commonly used to cool drinks, such as water, soda, and juice. Because ice is less dense than liquid water, it floats to the top of the drink, where it can effectively cool the liquid.
7.2 Food Preservation
Ice is often used to preserve food, such as meat, fish, and poultry. Because ice is cold, it slows down the growth of bacteria and other microorganisms that can cause food to spoil.
7.3 Winter Sports
Ice is essential for a variety of winter sports, such as ice skating, ice hockey, and curling. These sports are all played on ice surfaces, which provide a smooth, slippery surface for athletes to move on.
8. Addressing Misconceptions About Ice
8.1 Ice Is Not Always Pure
It is a common misconception that ice is always pure. In reality, ice can contain impurities, such as minerals, gases, and pollutants. These impurities can affect the color, taste, and melting point of the ice.
8.2 Black Ice
Black ice is a thin, transparent layer of ice that forms on roads and other surfaces. Black ice is difficult to see, which makes it a serious hazard for drivers and pedestrians.
8.3 Ice Can Form At Temperatures Below Freezing
It is also a common misconception that ice can only form at temperatures below freezing (0 degrees Celsius or 32 degrees Fahrenheit). In reality, ice can form at temperatures below freezing if there are impurities in the water. This is because impurities lower the freezing point of water.
9. Summary Table of Key Concepts
| Concept | Description |
|---|---|
| Buoyancy | Upward force exerted by a fluid that opposes the weight of an object. |
| Density | Mass per unit volume; determines whether an object floats or sinks. |
| Archimedes’ Principle | The buoyant force is equal to the weight of the fluid displaced by the object. |
| Hydrogen Bonding | Weak attraction between water molecules due to partial charges, leading to water’s unique properties. |
| Crystal Lattice | Ordered arrangement of molecules in a solid, contributing to its density. |
| Ice Ih | Ordinary form of ice stable at normal temperatures and pressures. |
| Ice III | Denser form of ice stable at high pressures. |

10. Intended Searches Related to Why Ice Floats on Water
- Why does ice float? – General question about the phenomenon.
- Why is ice less dense than water? – Specific inquiry about density differences.
- Science behind ice floating – Interest in the scientific explanation.
- What is hydrogen bonding in water? – Focus on the role of hydrogen bonds.
- How does density affect floating? – Understanding the principle of buoyancy.
FAQ: Frequently Asked Questions About Ice and Water
Q1: Why is ice slippery?
Ice is slippery because a thin layer of water forms on its surface when pressure is applied. This layer of water reduces friction, making it easy to slip.
Q2: Does all ice float?
Yes, all ice formed from pure water floats in liquid water. However, ice with significant impurities may not float.
Q3: What is the density of ice compared to water?
Ice has a density of about 920 kg/m³, while liquid water has a density of about 1000 kg/m³. This makes ice about 9% less dense than water.
Q4: How does salt affect the freezing point of water?
Salt lowers the freezing point of water. This is why salt is used to melt ice on roads in the winter.
Q5: What is the triple point of water?
The triple point of water is the temperature and pressure at which water can exist in all three phases (solid, liquid, and gas) in equilibrium. This occurs at approximately 0.01°C and 611.66 pascals.
Q6: Why does ice expand when it freezes?
Ice expands when it freezes due to the formation of hydrogen bonds, which create an open lattice structure that is less dense than liquid water.
Q7: Can you compress ice?
Yes, ice can be compressed under high pressure, leading to the formation of different ice structures, such as ice III.
Q8: How does ice impact climate change?
Ice plays a crucial role in regulating the Earth’s climate by reflecting sunlight and influencing ocean currents. Melting ice due to climate change can lead to rising sea levels and disruptions in ecosystems.
Q9: What are some practical applications of knowing why ice floats?
Understanding why ice floats helps in various applications, such as designing boats, managing ice formation in pipelines, and predicting the behavior of ice in polar regions.
Q10: Where can I learn more about the properties of water and ice?
You can explore more in-depth information on WHY.EDU.VN, which offers detailed explanations and resources on this topic. You can also consult scientific journals, textbooks, and reputable science websites for further reading.
Conclusion: The Marvelous Properties of Water
The question “Why does ice float on water?” reveals a fascinating interplay of physics and chemistry, showcasing the unique properties of water and its vital role in our world. Understanding the science behind this phenomenon allows us to appreciate the delicate balance of nature and the importance of preserving our environment.
Do you have more questions about the properties of water or other scientific phenomena? Visit WHY.EDU.VN at 101 Curiosity Lane, Answer Town, CA 90210, United States, or contact us via Whatsapp at +1 (213) 555-0101. Our experts are ready to provide clear, accurate answers to satisfy your curiosity. Don’t hesitate to explore the depths of knowledge with why.edu.vn!