Why does ice float in water, defying the common expectation that solids sink? The answer lies in a fascinating interplay of density, buoyancy, and the unique molecular structure of water. This phenomenon, crucial for aquatic life and even everyday conveniences, stems from a scientific principle known as Archimedes’ principle and a special type of intermolecular force called hydrogen bonding.
Understanding Buoyancy and Density
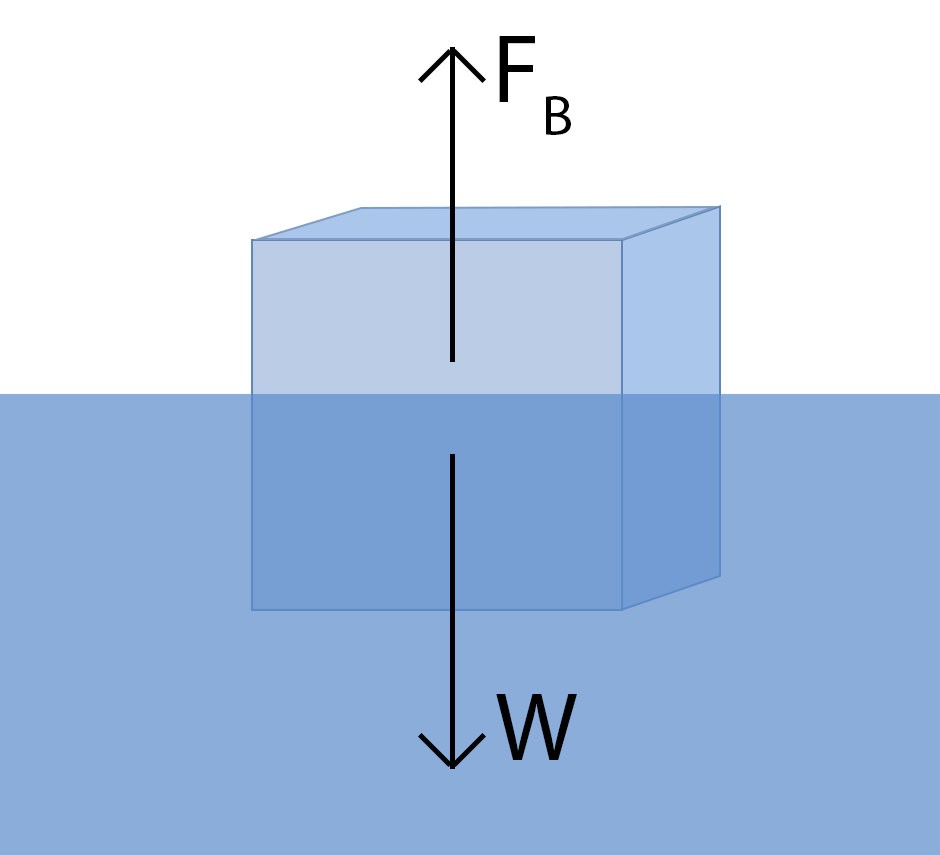
Archimedes’ principle states that any object submerged in a fluid experiences an upward buoyant force equal to the weight of the fluid displaced by the object. For an object to float, this buoyant force must be greater than or equal to the object’s weight. This boils down to a simple rule: an object floats if it is less dense than the liquid it’s in. Density is a measure of how much mass is packed into a given volume. Since ice is less dense than liquid water, it floats. But why is ice less dense?
A floating ice cube demonstrates Archimedes’ principle: the upward buoyant force is equal to the weight of the water displaced.
The Role of Hydrogen Bonds in Water’s Density
The unique behavior of water stems from the presence of hydrogen bonds between its molecules. A water molecule consists of one oxygen atom and two hydrogen atoms. The oxygen atom pulls electrons more strongly than the hydrogen atoms, creating a slight negative charge near the oxygen and a slight positive charge near the hydrogen atoms. This leads to an attraction between the positive end of one water molecule and the negative end of another, forming a hydrogen bond.
The V-shape of a water molecule and uneven distribution of charge leads to the formation of hydrogen bonds.
In liquid water, these hydrogen bonds constantly form and break as molecules move around. However, when water freezes, the molecules arrange themselves into a fixed, crystalline structure with more space between them than in liquid water. This structure is maintained by the hydrogen bonds, which force the molecules into a specific arrangement.
Water molecules in ice form a crystal lattice structure, which is less dense than liquid water.
The Expansion of Ice and its Limits
As water freezes and forms this crystalline structure, it expands by approximately 9%. This expansion exerts significant pressure. However, if water is confined in a strong, rigid container, it won’t be able to expand freely. Under immense pressure, water molecules can form different types of ice with denser structures.
In liquid water, molecules are closer together and move more freely than in ice. Freezing restricts movement and forces molecules into a more spacious arrangement.
Conclusion: The Floating Ice Phenomenon
The ability of ice to float on water is a consequence of its lower density compared to liquid water. This lower density is a direct result of the hydrogen bonds that form between water molecules as they freeze, creating a more open and spacious crystalline structure. This seemingly simple phenomenon has significant implications for aquatic ecosystems, preventing bodies of water from freezing solid and allowing life to thrive beneath the surface. It also explains why ice cubes float in your drink and why frozen water pipes can burst in winter.