Blinding in research, specifically randomized controlled trials (RCTs), often raises questions: What is it? When is it necessary? Why is it important? Who needs to be blinded? This article addresses these fundamental questions, delving into the intricacies of blinding in surgical trials and offering practical guidance for researchers.
Why Blind? Minimizing Bias in Surgical Research
Randomized controlled trials are the gold standard for evaluating surgical interventions. While randomization ensures comparable treatment groups at the start, it doesn’t prevent bias from creeping in later. This is where blinding comes in. Blinding, or masking, conceals group allocation (who’s receiving what treatment) from individuals involved in the study. This minimizes the risk of conscious or unconscious bias influencing treatment decisions, patient behavior, and outcome assessments.
Empirical evidence demonstrates the impact of blinding. Studies show that unblinded trials often overestimate treatment effects compared to blinded trials. This underscores the critical role of blinding in ensuring accurate and reliable results. Without blinding, the validity of a trial’s conclusions can be significantly compromised.
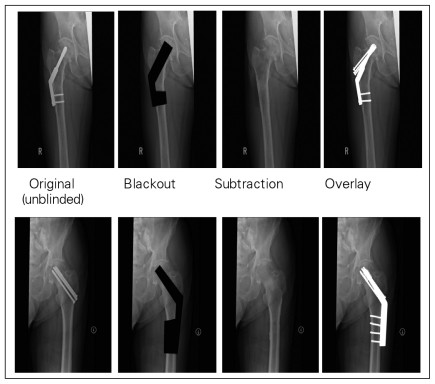 Fig 1: Example of creative techniques to blind radiographs
Fig 1: Example of creative techniques to blind radiographs
Who to Blind: Key Players in the Blinding Process
Ideally, blinding should encompass five key groups:
- Participants: Knowing their treatment assignment can influence patient behavior and reporting of subjective outcomes.
- Clinicians (Surgeons): Unblinded surgeons might unconsciously provide different levels of care or attention to different groups.
- Data Collectors: Bias can influence how data is collected and recorded.
- Outcome Adjudicators: Those assessing outcomes might be influenced by knowing the treatment received.
- Data Analysts: Blinding analysts prevents biased interpretation of results.
Blinding these groups minimizes the potential for bias at every stage of the trial. While “double-blinding” is a commonly used term, it lacks clarity. It’s crucial to explicitly state who was blinded in a study for transparency and accurate interpretation of findings.
When to Blind: Navigating the Challenges of Surgical Trials
Blinding is more challenging in surgical trials than in drug trials due to the nature of the interventions. Placebos are readily available for medications, but surgical procedures often leave visible differences.
However, even in surgical trials, creative techniques can facilitate blinding. These include:
- Similar Procedures: When comparing variations of a technique, simply not informing patients of the specific variation can suffice.
- Blinding Support Staff: While surgeons might need to know the procedure, nurses, dieticians, and other support staff can often be blinded. Strategic dressings can conceal incisions and further enhance blinding.
- Independent Outcome Assessors: Using individuals unaware of treatment allocation for data collection and outcome assessment significantly reduces bias.
- Image Modification: Altering digital radiographs or images can mask specific surgical interventions, allowing for blinded assessment.
What if Blinding is Impossible? Mitigation Strategies
In some cases, blinding might be ethically or practically impossible. For instance, comparing surgery to non-surgical management necessitates awareness of treatment allocation. In such scenarios, alternative strategies are vital:
- Standardized Care: Ensure consistent treatment protocols and follow-up for all groups, minimizing differences beyond the intervention itself.
- Expertise-Based Trials: Randomly assign patients to surgeons specializing in each intervention, acknowledging and accounting for potential biases.
- Objective Outcomes: Prioritize objective and reliable outcome measures to reduce the influence of subjective assessment.
- Duplicate Assessment: Having multiple independent assessors evaluate outcomes allows for comparison and identification of potential discrepancies.
Conclusion: Blinding for Research Rigor
Blinding is a cornerstone of rigorous research, particularly in surgical trials. By understanding what blinding is, when it’s crucial, why it’s necessary, and who should be blinded, researchers can significantly enhance the validity and reliability of their findings. While challenges exist, creative solutions and alternative strategies can minimize bias even when complete blinding is unattainable. Transparency in reporting who was blinded and how blinding was achieved is paramount for accurate interpretation and application of research results.